
(a)
Interpretation:
From the given repeating unit the structure of
Concept introduction:
The repeating unit describes the connectivity that occurs over and over (repeatedly) in a polymer. A new C-C single bond is formed using the bonds that are unaccounted for on either side of the given repeating unit. The number of repeating units determines the degree of
Answer to Problem 26.1P
From the given repeating unit the structure of polymer is
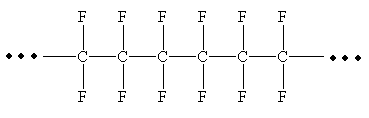
Explanation of Solution
The given repeating unit is
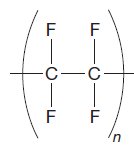
The repeating units are shown in parentheses. A new C-C single bond is formed using the bonds that are unaccounted for on either side of the given repeating unit. In the given repeating unit four F substituents are attached to the main repeating chain. As it is given that

From the given repeating unit the structure of polymer is drawn by joining repeating units by C-C single bond and the given n value.
(b)
Interpretation:
From the given repeating unit the structure of polymer having
Concept introduction:
The repeating unit describes the connectivity that occurs over and over (repeatedly) in a polymer. A new C-C single bond is formed using the bonds that are unaccounted for on either side of the given repeating unit. The number of repeating units determines the degree of polymerization (DP). DP is the value of the subscript n.
Answer to Problem 26.1P
From the given repeating unit the structure of polymer is
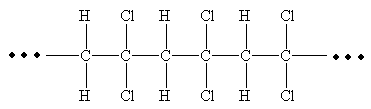
Explanation of Solution
The given repeating unit is
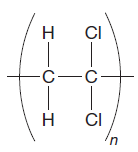
The repeating units are shown in parentheses. A new C-C single bond is formed using the bonds that are unaccounted for on either side of the given repeating unit. In the given repeating unit two Cl substituents are attached to the main repeating chain. As given that

From the given repeating unit the structure of polymer is drawn by joining repeating units by C-C single bond and the given n value.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forward
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





