
Interpretation:
The purine and pyrimidine ring structures should be drawn demonstrating the
Concept Introduction:
Heterocyclic
Explanation of Solution
Purine is represented in a double-ring structure which has four-nitrogen and five-carbon atoms. In this structure, carbon-2 and carbon-8 are obtained from N10 -formyl −H4folate and nitrogen-3 and nitrogen-9 are obtained from glutamine. There is no metabolic relation between purine and pyrimidines. Purine and Pyrimidines both have a different process for the synthesis and for the degradation. These both process of degradation and synthesis occur in all organisms. Structure of the purine is given below-
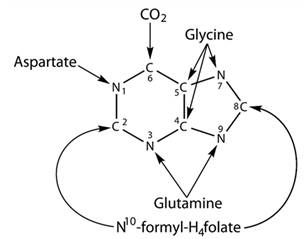
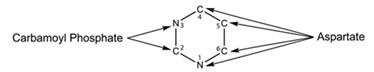
Pyrimidine is a six-membered single ring structure. It has two nitrogen and four carbon atoms. Nitrogen-3 and carbon-2 are obtained from carbamoyl phosphate. Carbon-4,5,6 and nitrogen-1 are obtained from aspartate. Structure for the pyrimidine is given below-
Want to see more full solutions like this?
Chapter 26 Solutions
Biochemistry
- If the pH of gastric juice is 1.6, what is the amount of energy (AG) required for the transport of hydrogen ions from a cell (internal pH of 7.4) into the stomach lumen? Assume that the membrane potential across this membrane is -70.0 mV and the temperature is 37 °C. AG= kJ mol-1arrow_forwardConsider the fatty acid structure shown. Which of the designations are accurate for this fatty acid? 17:2 (48.11) 18:2(A9.12) cis, cis-A8, A¹¹-octadecadienoate w-6 fatty acid 18:2(A6,9)arrow_forwardClassify the monosaccharides. H-C-OH H. H-C-OH H-C-OH CH₂OH H-C-OH H-C-OH H-C-OH CH₂OH CH₂OH CH₂OH CH₂OH D-erythrose D-ribose D-glyceraldehyde Dihydroxyacetone CH₂OH CH₂OH C=O Answer Bank CH₂OH C=0 HO C-H C=O H-C-OH H-C-OH pentose hexose tetrose H-C-OH H-C-OH H-C-OH aldose triose ketose CH₂OH CH₂OH CH₂OH D-erythrulose D-ribulose D-fructosearrow_forward
- Fatty acids are carboxylic acids with long hydrophobic tails. Draw the line-bond structure of cis-A9-hexadecenoate. Clearly show the cis-trans stereochemistry.arrow_forwardThe formation of acetyl-CoA from acetate is an ATP-driven reaction: Acetate + ATP + COA Acetyl CoA+AMP+ PP Calculate AG for this reaction given that the AG for the hydrolysis of acetyl CoA to acetate and CoA is -31.4 kJ mol-1 (-7.5 kcal mol-¹) and that the AG for hydrolysis of ATP to AMP and PP; is -45.6 kJ mol-1 (-10.9 kcal mol-¹). AG reaction kJ mol-1 The PP, formed in the preceding reaction is rapidly hydrolyzed in vivo because of the ubiquity of inorganic pyrophosphatase. The AG for the hydrolysis of pyrophosphate (PP.) is -19.2 KJ mol-¹ (-4.665 kcal mol-¹). Calculate the AG° for the overall reaction, including pyrophosphate hydrolysis. AGO reaction with PP, hydrolysis = What effect does the presence of pyrophosphatase have on the formation of acetyl CoA? It does not affect the overall reaction. It makes the overall reaction even more endergonic. It brings the overall reaction closer to equilibrium. It makes the overall reaction even more exergonic. kJ mol-1arrow_forwardConsider the Haworth projections of ẞ-L-galactose and ẞ-L-glucose shown here. OH CH₂OH OH CH₂OH OH OH OH ОН OH он B-L-galactose B-L-glucose Which terms describe the relationship between these two sugars? epimers enantiomers anomers diastereomersarrow_forward
- Classify each characteristic as describing anabolism or catabolism. Anabolism Answer Bank Catabolism transforms fuels into cellular energy, such as ATP or ion gradients uses NADPH as the electron carrier synthesizes macromolecules requires energy inputs, such as ATP uses NAD+ as the electron carrier breaks down macromoleculesarrow_forwardThe table lists the standard free energies (AG") of hydrolysis of some phosphorylated compounds. Compound kJ mol-1 kcal mol-1 Phosphoenolpyruvate (PEP) -61.9 -14.8 1,3-Bisphosphoglycerate (1,3-BPG) -49.4 -11.8 Creatine phosphate -43.1 -10.3 ATP (to ADP) -30.5 -7.3 Glucose 1-phosphate -20.9 -5.0 Pyrophosphate (PP) -19.3 -4.6 Glucose 6-phosphate -13.8 -3.3 Glycerol 3-phosphate -9.2 -2.2 What is the direction of each of the reactions shown when the reactants are initially present in equimolar amounts? (a) ATP + H2O ADP + P (b) ATP + glycerol glycerol 3-phosphate + ADParrow_forwardCharacterize each term or phrase as pertaining to simple or facilitated diffusion. Simple diffusion Facilitated diffusion Answer Bank requires an input of free energy lipophilic molecules directly through membrane via channels polar molecules Na+arrow_forward
- Sort the descriptions into properties that describe either saturated phospholipids or unsaturated phospholipids. Saturated phospholipids Saturated and unsaturated phospholipids Unsaturated phospholipids Answer Bank have no double bonds in the fatty acid carbon chains have straight fatty acid tails have at least one double bond in the fatty acid tails have bent fatty acid tails are built upon a glycerol backbone make the membrane somewhat rigid at low temperatures allow the membrane to remain fluid and flexible at low temperatures fatty acid tails pack tightly together maintain some space between adjacent phospholipidsarrow_forwardPlace the events of an action potential in order, starting and ending with a cell at its resting membrane potential. Cell starts at its resting membrane potential. Cell returns to its resting membrane potential. Answer Bank K+ channels fully open, and Na+ channels are inactivated. K* rushes out of the cell, causing repolarization. K+ channels close slowly, resulting in hyperpolarization. Na+ channel gates reset. Fast Na+ and slow K+ channels are activated. Na rushes into the cell, causing membrane depolarization. Ligand activation of the acetylcholine receptor depolarizes the membrane.arrow_forwardGlucose and fructose are reducing sugars. Sucrose, or table sugar, is a disaccharide consisting of both fructose and glucose. Is sucrose a reducing sugar? Why or why not? No, because only one anomeric carbon is involved in the glycosidic linkage. No, because both anomeric carbons are involved in the glycosidic linkage. Yes, because the fructose unit can convert to the open-chain form. Yes, because the glucose unit can convert to the open-chain form. Which statements about reducing sugars are true? The oxidation of a reducing sugar forms a carboxylic acid sugar. D-Arabinose (an aldose) is a reducing sugar. Reducing sugars contain keto groups instead of aldehyde groups. A disaccharide with its anomeric carbons joined by the glycosidic linkage cannot be a reducing sugar. A reducing sugar will not react with the Cu² + in Fehlings's reagent.arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





