
Concept explainers
a)
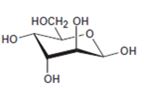
Interpretation:
The open-chain form of the sugar given is to be drawn.
Concept introduction:
The pyranose form is a cyclic hemiacetal form with a six membered ring formed by the nucleophilic addition of the –OH group on C5 to the C1 carbonyl group. The furanose form is a cyclic hemiacetal form with a five membered ring formed by the nucleophilic addition of the –OH group on C5 to the C2 carbonyl group.
The orientation of –OH group differs in α- and β- anomers. In α- anomer the OH on C1 is cis to the –OH at the lowest chirality center in Fischer projection while in β- anomer the –OH on C1 is trans to the –OH at the lowest chirality center in Fischer projection.
D sugars have the –O- at C5 on the right in the uncoiled form while L sugars have -O- at C5 on the left.
To draw:
The open-chain form of the sugar given.
b)
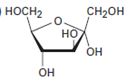
Interpretation:
The open-chain form of the sugar given is to be drawn.
Concept introduction:
The pyranose form is a cyclic hemiacetal form with a six membered ring formed by the nucleophilic addition of the –OH group on C5 to the C1 carbonyl group. The furanose form is a cyclic hemiacetal form with a five membered ring formed by the nucleophilic addition of the –OH group on C5 to the C2 carbonyl group.
The orientation of –OH group differs in α- and β- anomers. In the α- anomer the OH on C1 is cis to the –OH at the lowest chirality center in Fischer projection, while in β- anomer the –OH on C1 is trans to the –OH at the lowest chirality center in Fischer projection.
D sugars have the –O- at C5 on the right in the uncoiled form while L sugars have -O- at C5 on the left.
To draw:
The open-chain form of the sugar given.
c)
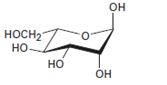
Interpretation:
The open-chain form of the sugar given is to be drawn.
Concept introduction:
The pyranose form is a cyclic hemiacetal form with a six membered ring formed by the nucleophilic addition of the –OH group on C5 to the C1 carbonyl group. The furanose form is a cyclic hemiacetal form with a five membered ring formed by the nucleophilic addition of the –OH group on C5 to the C2 carbonyl group.
The orientation of –OH group differs in α- and β- anomers. In the α- anomer the OH on C1 is cis to the –OH at the lowest chirality center in Fischer projection, while in β- anomer the –OH on C1 is trans to the –OH at the lowest chirality center in Fischer projection.
D sugars have the –O- at C5 on the right in the uncoiled form while L sugars have -O- at C5 on the left.
To draw:
The open-chain form of the sugar given.
Trending nowThis is a popular solution!

Chapter 25 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





