
(a)
Interpretation:
How to synthesize a given compound from
Concept introduction:
Answer to Problem 25.76P
The synthesis of a given compound from
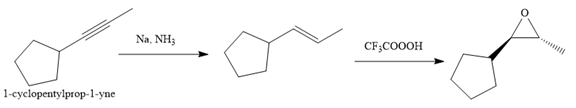
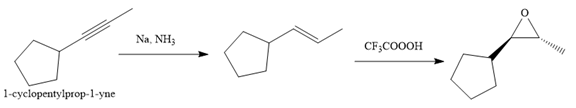
Explanation of Solution
The given synthesis is
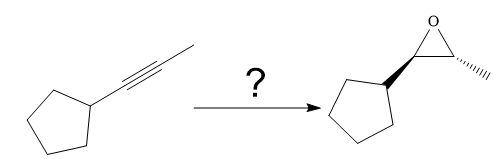
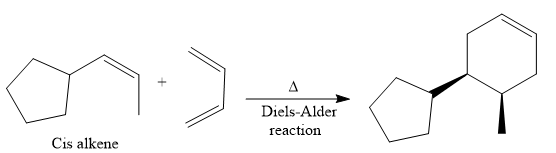
The direct conversion is not known. Therefore, the retrosynthesis analysis is done to know the possible route for the synthesis. It is noticed that the cyclopentyl ring and methyl group are trans to each other, thus the alkene results from
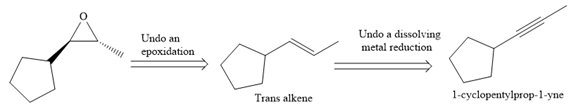
Thus the forward reaction is carried out as below:
Since the cyclopentyl ring and methyl group are trans to each other, the alkene results from
It is shown how to synthesize a given compound from
(b)
Interpretation:
It is to be shown how to synthesize a given compound from
Concept introduction:
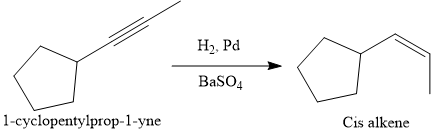
Alkynes can be converted to the corresponding alkene either by the treatment of alkali metal (e.g. Na, K) in ammonia or by catalytic hydrogenation. The advantage of the catalytic hydrogenation is to form the cis-alkene by the syn-addition of molecular hydrogen,
Answer to Problem 25.76P
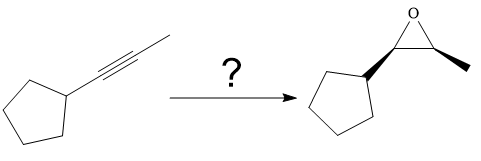
The synthesis of the given compound from
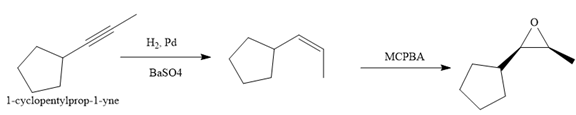
Explanation of Solution
The given synthesis is
The direct conversion is not known. Therefore, the retrosynthesis analysis is done to know the possible route for the synthesis. It is noticed that the cyclopentyl ring and methyl group are cis to each other, thus the alkene results from
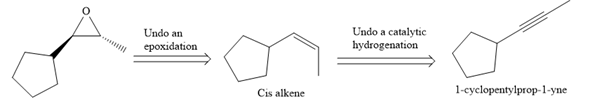
Thus the forward reaction is carried out as below:
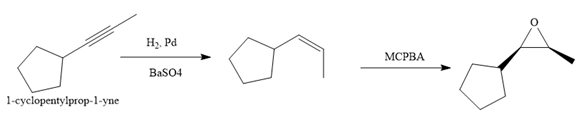
Since the cyclopentyl ring and methyl group are cis to each other, the alkene results from
It is shown how to synthesize a given compound from
(c)
Interpretation:
It is to be shown how to synthesize a given compound from
Concept introduction:
Alkynes can be converted to the corresponding alkene either by the treatment of alkali metal (e.g. Na, K) in ammonia called a dissolving metal reduction or by catalytic hydrogenation. The advantage of the catalytic hydrogenation is to form the cis-alkene by the syn-addition of molecular hydrogen,
Answer to Problem 25.76P
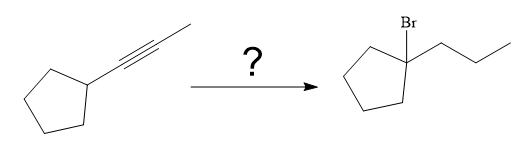
The synthesis of the given compound from
Explanation of Solution
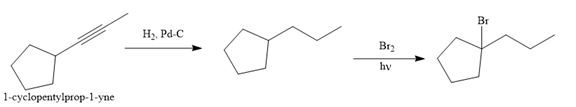
The given synthesis is
The direct conversion is not known. Therefore, the retrosynthesis analysis is done to know the possible route for the synthesis.
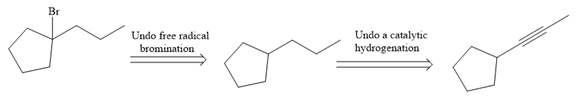
The forward reaction is carried out as:
In the first step the reduction of the alkyne is done to the corresponding alkane by the catalytic hydrogenation as follows:
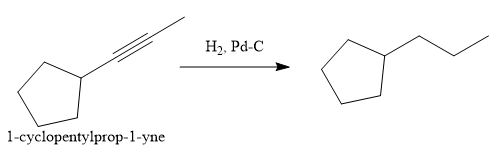
Finally, the proton of the tertiary carbon is replaced by bromine atom by the treatment of molecular bromine in the presence of light. The tertiary carbon is brominated via a free radical mechanism to form the required product.
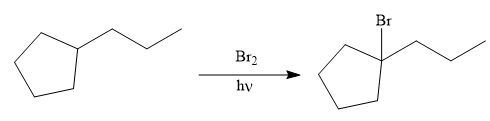
It is to be shown how to synthesize a given compound from
(d)
Interpretation:
How to synthesize a given compound from
Concept introduction:
Alkynes can be converted to the corresponding alkene either by the treatment of alkali metal (e.g. Na, K) in ammonia called a dissolving metal reduction or by catalytic hydrogenation. The advantage of the catalytic hydrogenation is to form the cis-alkene by the syn-addition of molecular hydrogen,
Answer to Problem 25.76P
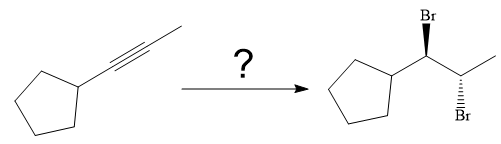
The synthesis of the given compound from
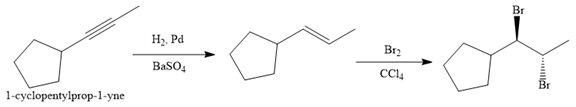
Explanation of Solution
The given synthesis is
The direct conversion is not known. Therefore, the retrosynthesis analysis is done to know the possible route for the synthesis.
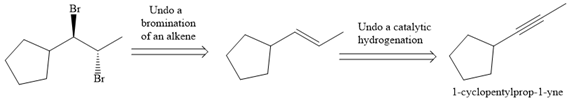
In the first step the reduction of the alkyne is done to the corresponding alkene by the catalytic hydrogenation as follows:
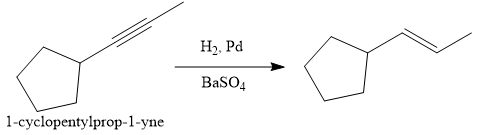
Finally, the addition of the molecular bromine is done to form the final, required product.
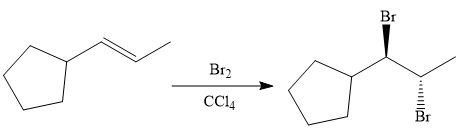
It is shown how to synthesize a given compound from
(e)
Interpretation:
How to synthesize a given compound from
Concept introduction:
Alkynes can be converted to the corresponding alkene either by the treatment of alkali metal (e.g. Na, K) in ammonia called a dissolving metal reduction or by catalytic hydrogenation. The advantage of the catalytic hydrogenation is to form the cis-alkene by the syn-addition of molecular hydrogen,
Answer to Problem 25.76P
The synthesis of the given compound from
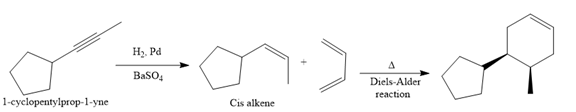
Explanation of Solution
The given synthesis is
The direct conversion is not known. Therefore, the retrosynthesis analysis is done to know the possible route for the synthesis.
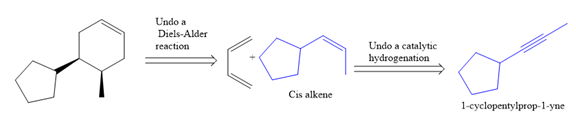
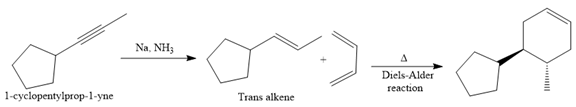
In the first step, the reduction of the alkyne is done to the corresponding alkene by the catalytic hydrogenation as follows:
Finally, a Diels-Alder reaction is carried out to produce the required product.
It is shown how to synthesize a given compound from
(f)
Interpretation:
It is to be shown how to synthesize a given compound from
Concept introduction:
Alkynes can be converted to the corresponding alkene either by the treatment of alkali metal (e.g. Na, K) in ammonia called a dissolving metal reduction or by catalytic hydrogenation. The advantage of the catalytic hydrogenation is to form the cis-alkene by the syn-addition of molecular hydrogen,
Answer to Problem 25.76P
The synthesis of the given compound from
Explanation of Solution
The given synthesis is
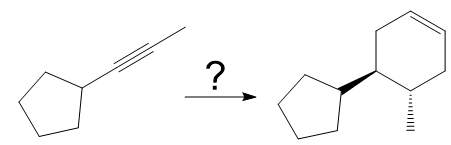
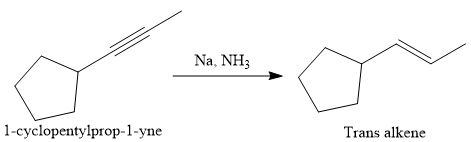
The direct conversion is not known. Therefore, the retrosynthesis analysis is done to know the possible route for the synthesis.
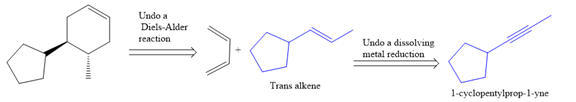
In the first step the reduction of the alkyne is done to the corresponding alkene by the catalytic hydrogenation as follows:
Finally, a Diels-Alder reaction is carried out to produce the required product.
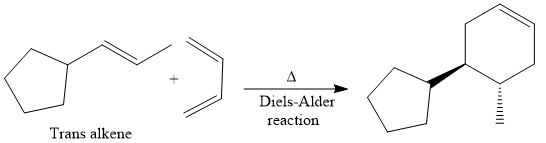
It is shown how to synthesize a given compound from
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Complete boxes in the flow chart. Draw the structure of the organic compound foundin each layer after adding 3M NaOH and extraction. Make sure to include any charges. Provide explanation on answers.arrow_forward== Vid4Q2 Unanswered ☑ Provide IUPAC name of product in the reaction below A 3,4-dimethylcyclohexene B 1,2-dimethylcyclohexane C 1,2-dimethylcyclohexene D 3,4-dimethylcyclohexane H₂ Pdarrow_forward5. Use the MS data to answer the questions on the next page. 14.0 1.4 15.0 8.1 100- MS-IW-5644 26.0 2.8 27.0 6.7 28.0 1.8 29.0 80 4.4 38.0 1.0 39.0 1.5 41.0 1.2 42.0 11.2 43.0 100.0 44.0 4.3 79.0 1.9 80.0 2.6 Relative Intensity 40 81.0 1.9 82.0 2.5 93.0 8.7 20- 95.0 8.2 121.0 2.0 123.0 2.0 136.0 11.8 0 138.0 11.5 20 40 8. 60 a. Br - 0 80 100 120 140 160 180 200 220 m/z Identify the m/z of the base peak and molecular ion. 2 b. Draw structures for each of the following fragments (include electrons and charges): 43.0, 93.0, 95.0, 136.0, and 138.0 m/z. C. Draw a reasonable a-fragmentation mechanism for the fragmentation of the molecular ion to fragment 43.0 m/z. Be sure to include all electrons and formal charges. 6. Using the values provided in Appendix E of your lab manual, calculate the monoisotopic mass for the pyridinium ion (CsH6N) and show your work.arrow_forward
- Nonearrow_forwardStereochemistry: Three possible answers- diastereomers, enantiomers OH CH₂OH I -c=0 21108 1101 41745 HOR CH₂OH IL Но CH₂OH TIL a. Compounds I and III have this relationship with each other: enantiomers b. Compounds II and IV have this relationship with each other: c. Compounds I and II have this relationship with each other: d. *Draw one structure that is a stereoisomer of II, but neither a diastereomer nor an enantiomer. (more than one correct answer)arrow_forwardNonearrow_forward
- Don't used Ai solutionarrow_forwardIn mass spectrometry, alpha cleavages are common in molecules with heteroatoms. Draw the two daughter ions that would be observed in the mass spectrum resulting from an alpha cleavage of this molecule. + NH2 Q Draw Fragment with m/z of 72arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





