
(a)
Interpretation:
The major product of the given reaction is to be predicted. A complete, detailed mechanism for the reaction is to be provided.
Concept introduction:
Halogen molecules undergo homolysis when irradiated with UV light to produce two radical atoms. Homolysis is the type of bond dissociation where each atom gets one of the bond electrons. An unpaired electron present in the radical of atom or molecule. It is a highly reactive and unstable species. This homolytic dissociation is called initiation.
Being reactive, a radical will react with other species present. This produces another radical, which can continue the reaction is a step called propagation. The reaction is generally branched as each initiation step produces two radicals. Propagation steps continue as long as radicals are present and may be terminated by a reaction between two radicals.
The reaction generally results in substitution, often of a hydrogen atom from the hydrocarbon skeleton. When there are distinct types of hydrogen atoms in the reactant, the hydrogen removed is one that will lead to the formation of the most stable radical. The relative stability of radicals changes in the same way as the stability of the carbocation. This decides the major product of the reaction.
Answer to Problem 25.40P
The major product of the reaction is
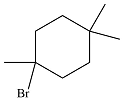
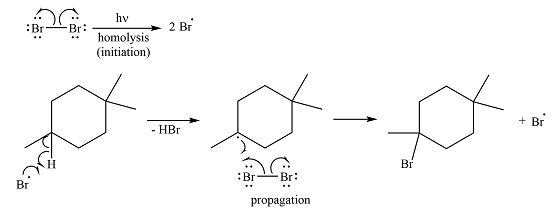
The complete mechanism of the reaction is
Explanation of Solution
The given reaction is
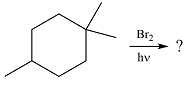
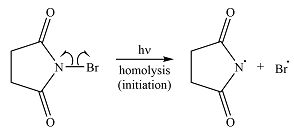
Irradiation of the reaction mixture with UV light leads to the formation of two bromine radicals through homolysis in the initiation step.
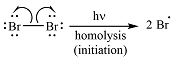
One of the bromine radicals reacts with the hydrocarbon and extracts a hydrogen atom (radical). There are three distinct types of – hydrogen atoms in the substrate; therefore three types of radicals can be produced in this step –
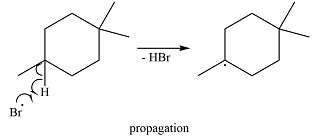
The
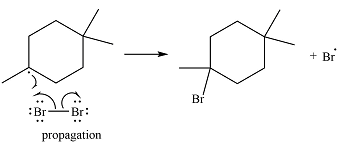
The bromine radical can continue the radical reaction chain or combine with another alkyl or bromine radical, in which case, the particular chain is terminated.
Thus, the complete reaction mechanism for the reaction can be drawn as

The major product of reaction will be

The major product of the reaction is determined on the basis of the stability of the alkyl radical produced after the initiation step.
(b)
Interpretation:
The major product of the given reaction is to be predicted. A complete, detailed mechanism for the given reaction is to be provided.
Concept introduction:
Halogen molecules undergo homolysis when irradiated with UV light to produce two radical atoms. Homolysis is the type of bond dissociation where each atom gets one of the bond electrons. An unpaired electron present in the radical of atom or molecule. It is a highly reactive and unstable species. This homolytic dissociation is called initiation.
Being reactive, a radical will react with other species present. This produces another radical, which can continue the reaction is a step called propagation. The reaction is generally branched as each initiation step produces two radicals. Propagation steps continue as long as radicals are present and may be terminated by a reaction between two radicals.
The reaction generally results in substitution, often of a hydrogen atom from the hydrocarbon skeleton. When there are distinct types of hydrogen atoms in the reactant, the hydrogen removed is one that will lead to the formation of the most stable radical. The relative stability of radicals changes in the same way as the stability of the carbocation. This decides the major product of the reaction.
Answer to Problem 25.40P
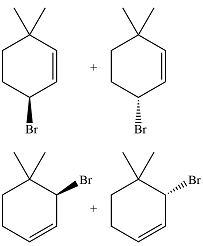
The major product of the reaction is a mixture of four isomers:
The complete mechanism for the reaction is
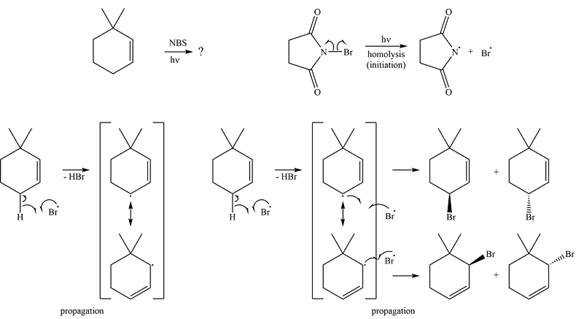
Explanation of Solution
The given reaction is
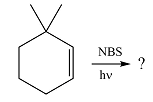
Irradiation of NBS (
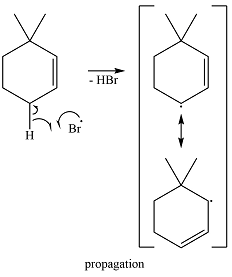
The bromine radical reacts with the substrate in the first of the propagation steps to produce the most stable radical. The most stable alkyl radical that can be produced on the extraction of a hydrogen atom is the allylic radical. Therefore, the hydrogen in the allylic position is extracted preferentially. The allylic radical is resonance stabilized.
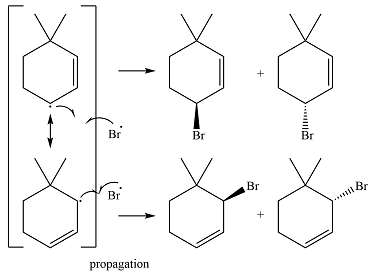
In the next propagation step, each resonance form of the allylic radical reacts with another bromine radical to produce the product. Since the cyclic radical is unsymmetric, each one produces a mixture of two isomers, giving a total of four isomeric products.
The complete mechanism for the reaction can be drawn as
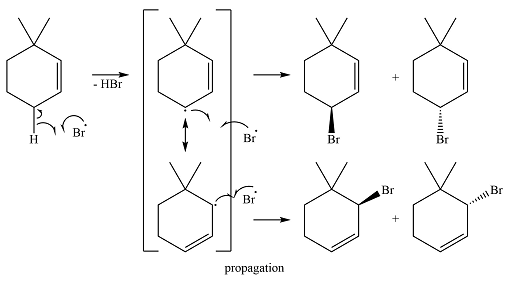
Thus the major product of the reaction is a mixture of four isomers.
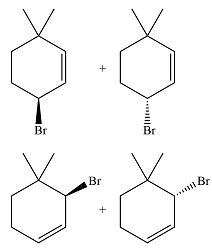
The major product of the reaction is determined on the basis of the stability of the alkyl radical produced after the initiation step.
(c)
Interpretation:
The major product of the given reaction is to be predicted. A complete, detailed mechanism for the given reaction is to be provided.
Concept introduction:
Under conditions that do not result in homolysis, halogen molecules, particularly chlorine and bromine, behave as electrophiles if electron-rich species such as
The addition is a two-step reaction. In the first step, a cyclic halonium intermediate is produced. In the second step, the halide ion formed in the first step acts as a nucleophile and adds to one of the carbons of the halonium intermediate to produce the vicinal dihalide product. Since the cyclic halonium intermediate is planar, the halide addition in the second step can occur from above or below the plane of the ring, giving a mixture of two isomers if the
Answer to Problem 25.40P
The major product of the reaction is a mixture of vicinal dibromo isomers.
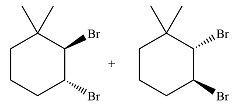
The whole mechanism of the reaction is
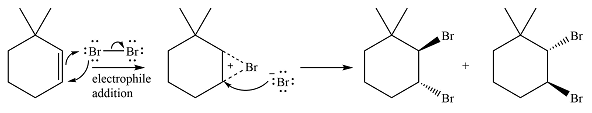
Explanation of Solution
The given reaction is
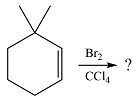
In the absence of UV irradiation, bromine will not undergo homolysis to produce radicals. Therefore, the reaction will be a simple electrophilic addition.
The
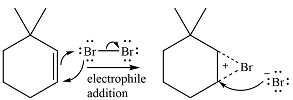
In the second step, the bromide ion produced in the first step will add to one of the carbons of the bromonium ring. Since the bromonium ring is planar, this addition can be from above or below the plane of the ring. This will lead to the formation of a mixture of isomers.
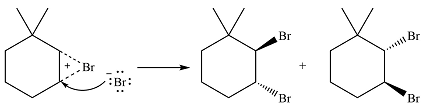
Thus, the whole mechanism for the reaction can be drawn as
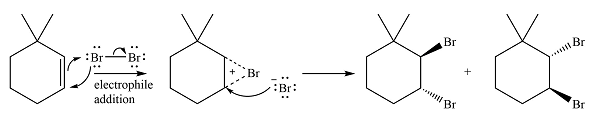
The major product of the reaction will be
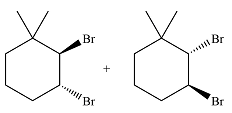
The major product of the reaction is determined on the basis of an electrophilic addition of the bromine molecule to the double bond.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardreciprocal lattices rotates along with the real space lattices of the crystal. true or false?arrow_forwardDeducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forward
- Predict the major products of the following organic reaction: Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Larrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forwardPredict the major products of the following organic reaction: O O + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. eserved. Terms of Use | Privacy Center >arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





