
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
16th Edition
ISBN: 9781323406038
Author: McMurry
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25, Problem 25.11UKC
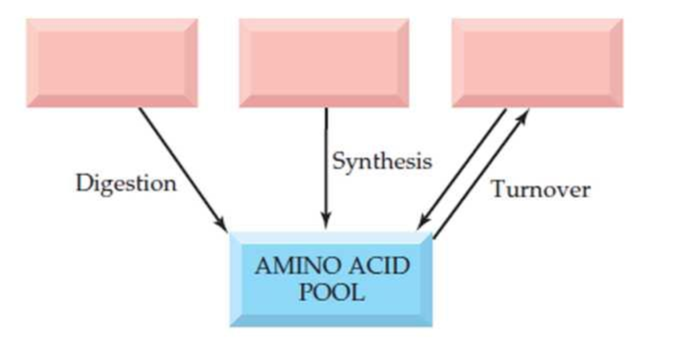
In the diagram shown here, fill in the sources for the amino acid pool.
Expert Solution & Answer
Interpretation Introduction
Interpretation:
The missing sources for the amino acids pool has to be filled.
Concept introduction:
- Amino acid had both amino functional group and carboxyl functional group in a molecule.
- Amino acid pool is the entire collection of free amino acids in the whole body.
Explanation of Solution
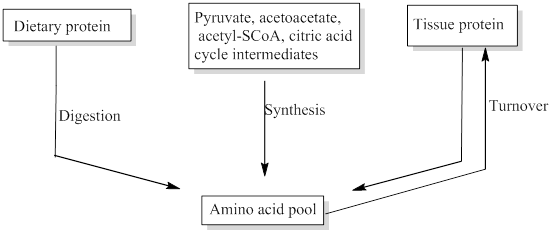
Free amino acids are present throughout the body, in cells and the extracellular fluids. This pool is supplied by few sources include,
- Non-protein nitrogen compounds
- Dietary protein
- Tissue protein etc.
Conclusion
The missing sources for the amino acids pool was filled.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
Show the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.
Label the following polysaccharide derivatives as reducing or nonreducing.
a.
C.
b.
HO
CH₂OH
CH2OH
OH
OH
OH
OH
OH
HOCH₂
OH
OH
OH
HOCH₂
HO
HO
HO
OH
OH
ΙΟ
CH₂OH
OH
OH
"OH
OH
For a red blood cell (erythrocyte) undergoing active glycolysis, briefly explain how
increases in concentration of the following factors are likely to affect glycolytic flux.
a. ATP
b. AMP
c. F-1,6-BP
d. F-2,6-BP
e. Citrate
f. Glucose-6-phosphate
Chapter 25 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
Ch. 25.2 - Prob. 25.1PCh. 25.2 - Prob. 25.2KCPCh. 25.3 - Prob. 25.3PCh. 25.3 - Prob. 25.4PCh. 25.3 - Prob. 25.5PCh. 25.3 - Prob. 25.6PCh. 25.4 - Prob. 25.1CIAPCh. 25.4 - Prob. 25.2CIAPCh. 25.4 - Prob. 25.3CIAPCh. 25.4 - Prob. 25.7P
Ch. 25.4 - Prob. 25.8KCPCh. 25.6 - Prob. 25.9PCh. 25.6 - Prob. 25.10KCPCh. 25.6 - What is meant by a conditional amino acid?Ch. 25.6 - Prob. 25.5CIAPCh. 25.6 - Prob. 25.6CIAPCh. 25 - In the diagram shown here, fill in the sources for...Ch. 25 - Prob. 25.12UKCCh. 25 - Prob. 25.13UKCCh. 25 - Prob. 25.14UKCCh. 25 - Prob. 25.15UKCCh. 25 - Prob. 25.16UKCCh. 25 - Prob. 25.17APCh. 25 - Prob. 25.18APCh. 25 - Prob. 25.19APCh. 25 - Prob. 25.20APCh. 25 - Prob. 25.21APCh. 25 - Prob. 25.22APCh. 25 - What is the structure of the -keto acid formed...Ch. 25 - Prob. 25.24APCh. 25 - In general, how does oxidative deamination differ...Ch. 25 - Prob. 25.26APCh. 25 - Prob. 25.27APCh. 25 - Prob. 25.28APCh. 25 - Prob. 25.29APCh. 25 - Prob. 25.30APCh. 25 - Prob. 25.31APCh. 25 - Prob. 25.32APCh. 25 - Prob. 25.33APCh. 25 - Prob. 25.34APCh. 25 - How do essential and nonessential amino acids...Ch. 25 - Prob. 25.36APCh. 25 - Prob. 25.37APCh. 25 - How is tyrosine biosynthesized in the body? What...Ch. 25 - Prob. 25.39APCh. 25 - Prob. 25.40APCh. 25 - Prob. 25.41APCh. 25 - What energy source is used in the formation of...Ch. 25 - Write the equation for the transamination reaction...Ch. 25 - Prob. 25.44CPCh. 25 - Prob. 25.45CPCh. 25 - Prob. 25.46CPCh. 25 - Prob. 25.47CPCh. 25 - Prob. 25.48CPCh. 25 - Prob. 25.49CPCh. 25 - Prob. 25.50CPCh. 25 - Prob. 25.51CPCh. 25 - Prob. 25.52CPCh. 25 - Why might it be a bad idea to take large...Ch. 25 - Prob. 25.54GPCh. 25 - Prob. 25.55GPCh. 25 - Prob. 25.56GP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- The ∆G°’ for hydrolysis of phosphoenol pyruvate is -62.2 kJ/mol. The standard freeenergy of ATP hydrolysis is -30.5 kJ/mol. A. What is the standard free energy and K eq of the spontaneous reaction betweenADP/ATP and phosphoenol pyruvate. B. Repeat A for F-1,6-BP (∆G°’=-16.7 kJ/mol) and 1,3-BPG (∆G°’=-49.6 kJ/mol)hydrolysis. C. If the ATP and ADP concentrations are 8mM and 1mM respectively, what would bethe ratio of pyruvate/phosphoenolpyruvate at equilibrium?arrow_forwardAnswerarrow_forward13. Which one is the major organic product of the following sequence of reactions? A OH (CH3)2CHCH2COOH SOCI2 CH3OH 1. CH3MgBr 2. H₂O, H+ B C D OH E OHarrow_forward
- 14. Which one is the major organic product of the following sequence of reactions? (CH3)2CH-COCI CH3OH 1. DIBALH, -78°C 1. PhCH2MgBr ? 2. H2O, HCI 2. H2O, HCI OH OMe A Ph B Ph OH Ph C OMe Ph D E OH .Pharrow_forward6. Which one is the major organic product obtained from the following reaction? CO₂Me 1. LiAlH4 2. H₂O CH₂OH CH₂OCH3 5555 HO A B HO C HO D CH₂OH E ?arrow_forward1. (10 points) Pulverized coal pellets, which may be ° approximated as carbon spheres of radius r = 1 mm, are burned in a pure oxygen atmosphere at 1450 K and 1 atm. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C + O₂ →> CO₂. The reaction rate is first order and of the form No2 = k₁C₁₂(r), where k₁ = 0.1 m/s. Neglecting changes in r, determine the steady-state O₂ molar consumption rate in kmol/s. At 1450 K, the binary diffusion coefficient for O2 and CO2 is 1.71 x 10ª m²/s.arrow_forward
- 2. (20 points) Consider combustion of hydrogen gas in a mixture of hydrogen and oxygen adjacent to the metal wall of a combustion chamber. Combustion occurs at constant temperature and pressure according to the chemical reaction 2H₂+ O₂→ 2H₂O. Measurements under steady-state conditions at 10 mm from the wall indicate that the molar concentrations of hydrogen, oxygen, and water vapor are 0.10, 0.10, and 0.20 kmol/m³, respectively. The generation rate of water vapor is 0.96x102 kmol/m³s throughout the region of interest. The binary diffusion coefficient for each of the species (H, O̟, and H₂O) in the remaining species is 0.6 X 10-5 m²/s. (a) Determine an expression for and make a qualitative plot of C as a function of distance from the wall. H2 (b) Determine the value of C2 at the wall. H2 (c) On the same coordinates used in part (a), sketch curves for the concentrations of oxygen and water vapor. This will require you to calculate Co, and C. 02 H20 (d) What is the molar flux of water…arrow_forward4. (15 points) Consider a spherical organism of radius ro within which respiration occurs at a uniform volumetric rate of That is, oxygen (species A) consumption is governed by a first- order reaction, homogeneous chemical reaction. a. If a molar concentration of CA(ro) = CA,o is maintained at the surface of the organism, obtain an expression for the radial distribution of oxygen, CA(r), within the organism. Hint: To simplify solution of the species diffusion equation, invoke the transformation y = rCA. b. Obtain an expression for the rate of oxygen consumption within the organism. c. Consider an organism of radius ro = 0.10 mm and a diffusion coefficient of DAB = 108 m2/s. If CA, o = 5 x105 kmol/m3 and k1 20 s1, estimate the corresponding value of the molar concentration at the center of the organism. What is the rate of oxygen consumption by the organism?arrow_forward3. (15 points) Living cells homogeneously distributed (immobilized) with an agarose gel require glucose to survive. An important aspect of the biochemical system design is the effective diffusion coefficient of glucose (A) into the cell- immobilized gel. Consider the experiment shows below where a slab of the cell-immobilized gel of 1.0cm thickness is placed within a well-mixed aqueous solution of glucose maintained at a concentration of 50 mmol/L. The glucose consumption within the cell-immobilized gel proceeds by a zero-order process given by R₁ = -0.05 mmol/(L min). The solubilities of glucose in both the water and the gel are the same; that is, the concentration of the glucose on the water side of the water-gel interface is equal to the concentration of the glucose on the gel side of the water gel interface. A syringe is mounted at the center of the gel carefully excises a tiny sample of the gel for glucose analysis. A Well mixed solution Constant concentration 50nmol/L Living…arrow_forward
- Two tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu Can you please help show the titration curve for both of these peptides and calculate the PI?arrow_forwardTwo tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu What is the structure of the PTH derivative produced during the last round of amino acid sequencing?arrow_forwardWhat is the primary sequence of this undecapeptide? Also, if x-ray crystallography shows a highly stable hairpin turn within the polypeptide, what about the primary sequence explains this structural feature?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning


Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning


Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning
The Cell Membrane; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=AsffT7XIXbA;License: Standard youtube license