
(a)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in
Answer to Problem 24.57P
The structure of the product of the given reaction is
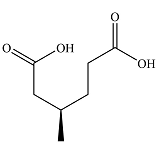
Explanation of Solution
The given reaction is
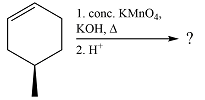
Hot, concentrated permanganate (
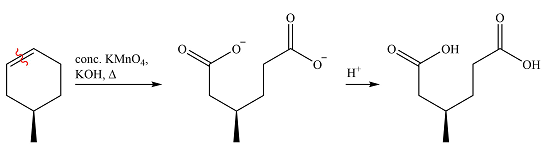
Therefore, the product of the reaction is

The products of the reaction were determined on the basis of the cleavage of the double bond by hot concentrated permanganate in basic medium and the oxidation of the two carbons to carbonyl groups.
(b)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes and alkynes are cleaved when treated with hot, concentrated permanganate in a basic solution in an oxidative cleavage reaction. Both carbons of the alkyne are oxidized in the process to carboxyl groups. The end carbon of a terminal alkyne is oxidized completely to carbon dioxide. The final functional group that is formed depends on the reaction conditions. The permanganate ion is added across the multiple bonds in a way similar to the Diels-Alder reaction, a
Answer to Problem 24.57P
The structure of the organic product of the given reaction is
Explanation of Solution
The given reaction is

Hot, concentrated permanganate (
![]()
Therefore, the organic product of the reaction is

The products of the reaction were determined on the basis of the cleavage of the triple bond by hot concentrated permanganate in basic medium and the oxidation of the internal carbon to carboxylic acid and the terminal carbon to carbon dioxide.
(c)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes are cleaved when treated with ozone in a reaction called ozonolysis. Both carbons of the alkene are oxidized in the process to carbonyl groups. Ozone adds across the double bond in a
Answer to Problem 24.57P
The structures of the products of the given reaction are
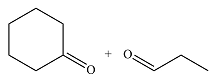
Explanation of Solution
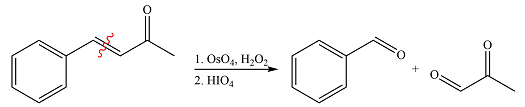
The given reaction is
Ozone will add across the double bond to initially form a molozonide. The molozonide rearranges to an ozonide following a cycloelimination and a cycloaddition. The ozonide is then reduced by zinc-acetic acid to cyclohexanone and propanal.
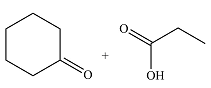
Therefore, the products of the reaction are

The products of the ozonolysis reaction were determined on the basis of cleavage of the double bond and oxidation of the two carbons to carbonyl groups.
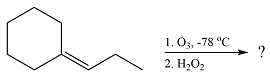
(d)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes are cleaved when treated with ozone in a reaction called ozonolysis. Both carbons of the alkene are oxidized in the process to carbonyl groups. Ozone adds across the double bond in a
Answer to Problem 24.57P
The structures of the organic products of the given reaction are
Explanation of Solution
The given reaction is
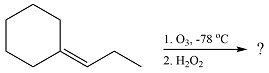
Ozone will add across the double bond to initially form a molozonide. The molozonide rearranges to an ozonide following a cycloelimination and a cycloaddition. One of the carbons of the double bond is oxidized initially to an aldehyde which is further oxidized to a carboxylic acid on treatment with
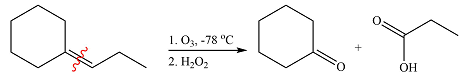
Therefore, the products of the reaction are

The products of the ozonolysis reaction were determined on the basis of cleavage of the double bond and oxidation of the two carbons to carbonyl groups.
(e)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes and alkynes are cleaved when treated with osmium tetroxide and
Answer to Problem 24.57P
The structures of the organic products of the given reaction are
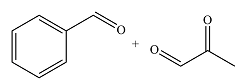
Explanation of Solution
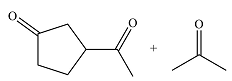
The given reaction is
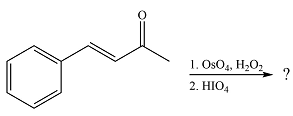
Osmium tetroxide adds across the double bond. Hydrolysis of the adduct yields a
Therefore, the products of the reaction are

The products of the reaction were determined on the basis of addition of
(f)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes and alkynes are cleaved when treated with hot, concentrated permanganate in a basic solution in an oxidative cleavage reaction. Both carbons of the alkene or alkyne are oxidized in the process to carbonyl groups or carbon dioxide. The final functional group that is formed depends on the reaction conditions. The permanganate ion is added across the multiple bonds in a way similar to the Diels-Alder reaction, a
Answer to Problem 24.57P
The structures of the organic products of the given reaction are
Explanation of Solution
The given reaction is
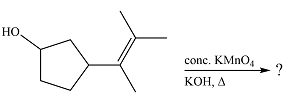
Hot, concentrated permanganate (
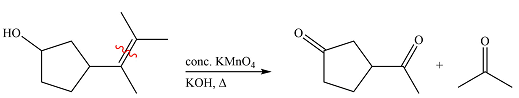
Therefore, the product of the reaction is

Hot concentrated permanganate in basic medium oxidized the double-bonded carbons of an alkene to carbonyl groups.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- for this question. Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!! THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!! I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!! First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion HOMEWORK, NOT EXAM!! ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!arrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forward
- Use the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forwardCan I please get help with this? And can I please the lowest possible significant number?arrow_forward
- What is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forwardwhat is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forward
- One step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forwardWhy does the following reaction lead to poor yields? Correct the reaction. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





