
(a)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in
Answer to Problem 24.58P
The structure of the reactant in the given reaction is
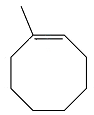
Explanation of Solution
The given reaction is
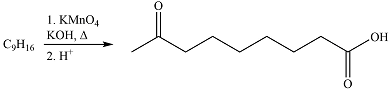
Hot, concentrated permanganate (
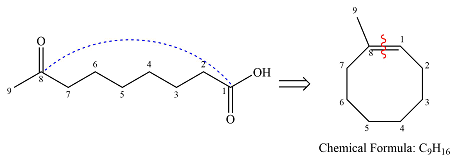
Therefore, the structure of the reactant must be

The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
(b)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes and alkynes are cleaved when treated with hot, concentrated permanganate in a basic solution in an oxidative cleavage reaction. Both carbons of the alkyne are oxidized in the process to carboxyl groups. The end carbon of a terminal alkyne is oxidized completely to carbon dioxide. The final functional group that is formed depends on the reaction conditions. The permanganate ion is added across the double bond in a way similar to the Diels-Alder reaction, a
Answer to Problem 24.58P
The structure of the reactant in the given reaction is
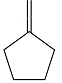
Explanation of Solution
The given reaction is
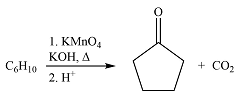
Hot, concentrated permanganate (
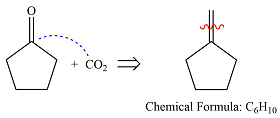
Therefore, the structure of the reactant must be

The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
(c)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes are cleaved when treated with ozone in a reaction called ozonolysis. Both carbons of the alkene are oxidized in the process to carbonyl groups. Ozone adds across the double bond in a
Answer to Problem 24.58P
The structure of the reactant in the given reaction is
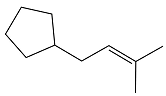
Explanation of Solution
The given reaction is
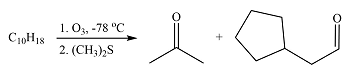
Ozonolysis will cleave the double bond in the reactant, oxidizing the two carbons to carbonyl groups. Therefore, the two carbonyl carbons in the products must have been joined by a double bond in the reactant.
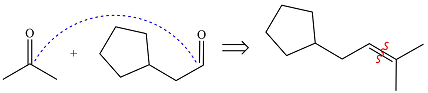
Therefore, the structure of the reactant must be

The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
(d)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes are cleaved when treated with ozone in a reaction called ozonolysis. Both carbons of the alkene are oxidized in the process to carbonyl groups. Ozone adds across the double bond in a
Answer to Problem 24.58P
The structures of the reactant in the given reaction is
![]()
Explanation of Solution
The given reaction is
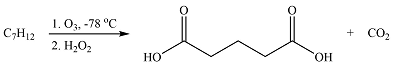
Ozonolysis will cleave a double bond in the reactant to oxidize the two carbons to carbonyl groups. If the product is an aldehyde, it is oxidized to the carboxylic acid group by
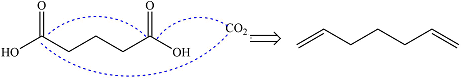
Therefore, the structure of the reactant must be
![]()
The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Consider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part 1 of 2 Br CH,CN + I¯ What is the correct mechanism for the reaction? Select the single best answer. @SN2 ○ SN 1 Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. X હૈarrow_forward20.33 Think-Pair-Share (a) Rank the following dienes and dienophiles in order of increasing reactivity in the Diels-Alder reaction. (i) CO₂Et (ii) COEt || CO₂Et MeO MeO (b) Draw the product that results from the most reactive diene and most reactive dienophile shown in part (a). (c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc- curs between the diene and dienophile in part (b). (d) Is the major product formed in part (b) the endo or exo configuration? Explain your reasoning.arrow_forward20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a tricyclic product. Propose a structural formula for the product. CN heat An intramolecular Diels-Alder adductarrow_forward
- What is the reaction mechanism for this?arrow_forwardWhat is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forward
- What is the reaction mechanism for this?arrow_forward20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





