
Concept explainers
(a)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
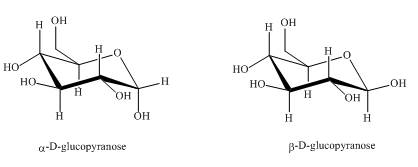
Explanation of Solution
The compounds,
They are diastereomers also as they are not mirror images of each other. The structure of both the compounds is shown below.

Figure 1
These two compounds are anomers as well as diastereomers as shown in Figure 1.
(b)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
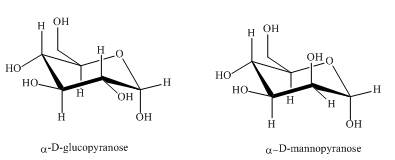
Explanation of Solution
Both the above structures of

Figure 2
The compounds
(c)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
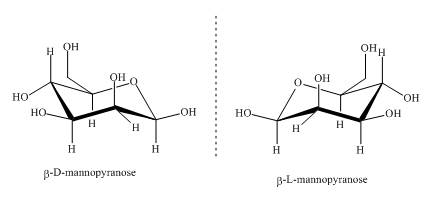
Explanation of Solution
Sugars can be divided into two groups based on the symmetry of carbon atoms. If the molecule has asymmetric carbon atom it will have a non superimposable mirror image. The non superimposable mirror images are known as enantiomers. As the given compounds are non super imposable mirror images of each other they are enantiomers. This can be well explained by the illustrations shown below.

Figure 3
These two compounds
(d)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
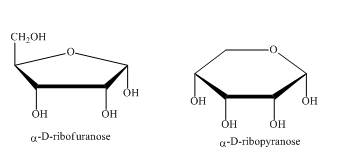
Explanation of Solution
As the isomers are the compounds, having similar chemical formula but different structures. These two compounds have same chemical formula. The chemical structure is of these two compounds is quite different as shown in Figure 4.

Figure 4
In the above shown compounds, one is a pentose sugar furanose while the other is a hexose sugar pyranose.
These two compounds,
(e)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
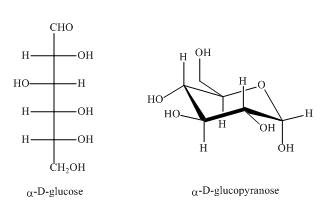
Explanation of Solution
As the isomers are the compounds, having similar chemical formula but different structures. Both the above structures are constitutional isomers as shown below in the Figure.

Figure 5
In the above shown compounds, one is a ring structure having keto group while the other is open ring structure having aldehyde as the
The given compounds
(f)
Interpretation:
Whether the compounds,
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons. The naming of the same compound can be done in different manners.
Answer to Problem 24.39AP
The compounds,
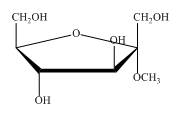
Explanation of Solution
The structure of

Figure 6
So, the compound is same, they are identical. In first name it is taken as methyl derivative of
The compound shown in Figure 6 can be named as
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry Study Guide and Solutions
- 7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forwardAssign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forward
- Please help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forward
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


