
Concept explainers
(a)
Interpretation:
The products expected when D-ribose is reacted with dilute ![]() is to be stated.
is to be stated.
Concept introduction:
The
Answer to Problem 24.35AP
The product obtained when D-ribose is reacted with dilute ![]() is shown below.
is shown below.
Explanation of Solution
The product obtained when D-ribose is reacted with dilute ![]() is shown below.
is shown below.

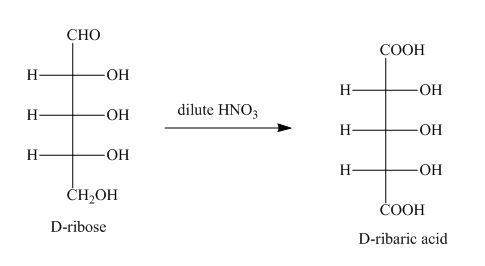
Figure 1
The oxidation of D-ribose into D-ribaric acid occurs in the presence of dilute nitric acid.
The product obtained when D-ribose is reacted with dilute ![]() is shown in Figure 1.
is shown in Figure 1.
(b)
Interpretation:
The products expected when D-ribose is reacted with ![]() is to be stated.
is to be stated.
Concept introduction:
Kiliani-Fischer process is is the reaction pathway by which an aldose is extended by one carbon unit. The first step of this reaction is the attack of the cyanide group on the carbonyl carbon of the aldehyde group resulting in the formation of the cyanohydrins. The cyanohydrins thus formed is reduced to imine with catalytic hydrogenation. The imine thus formed can easily be hydrolyzed by into aldose and ammonium ion.
Answer to Problem 24.35AP
The products obtained when D-ribose is reacted with ![]() are shown below.
are shown below.
Explanation of Solution
The products obtained when D-ribose is reacted with ![]() are shown below.
are shown below.

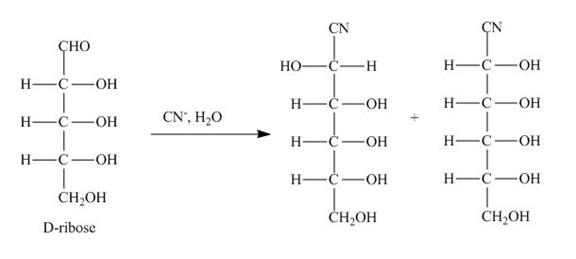
Figure 2
The D-ribose is converted into cyanohydrins by the nucleophilic attack of the cyanide group on the carbonyl carbon of the aldehyde group.
The products obtained when D-ribose is reacted with ![]() are shown in Figure 2.
are shown in Figure 2.
(c)
Interpretation:
The products expected when the product of part (b) is reacted with ![]() and
and ![]() is to be stated.
is to be stated.
Concept introduction:
Kiliani-Fischer process is is the reaction pathway by which an aldose is extended by one carbon unit. The first step of this reaction is the attack of the cyanide group on the carbonyl carbon of the aldehyde group resulting in the formation of the cyanohydrins. The cyanohydrins thus formed is reduced to imine with catalytic hydrogenation. The imine thus formed can easily be hydrolyzed by into aldose and ammonium ion.
Answer to Problem 24.35AP
The products obtained when the product of part (b) is reacted with ![]() and
and ![]() are shown below.
are shown below.
Explanation of Solution
The products obtained when the product of part (b) is reacted with ![]() and
and ![]() are shown below.
are shown below.

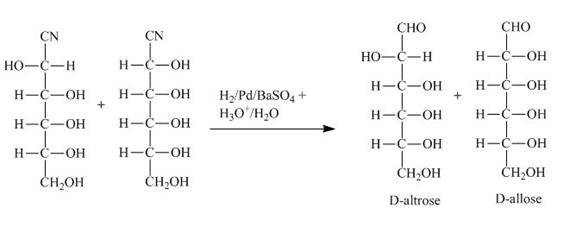
Figure 3
The product of part (b) is the cyanohydrin of D-ribose which is then converted into the extended aldose, altrose and allose. The catalytic hydrogenation of cyanohydrin into imine is done by ![]() . The imine then formed is hydrolyzed into the altrose and allose by
. The imine then formed is hydrolyzed into the altrose and allose by ![]() .
.
The products obtained when the product of part (b) is reacted with ![]() and
and ![]() are shown in Figure 3.
are shown in Figure 3.
(d)
Interpretation:
The products expected when D-ribose is reacted with ![]() is to be stated.
is to be stated.
Concept introduction:
A monosaccharide is converted into cyclic acetals on reaction with alcohols in the presence of acidic conditions. The hydroxide group right to the oxygen atom in the pyranose ring structure is methylated and result in the formation of acetal.
Answer to Problem 24.35AP
The product obtained when D-ribose is reacted with ![]() is shown below.
is shown below.
Explanation of Solution
The product obtained when D-ribose is reacted with ![]() is shown below.
is shown below.
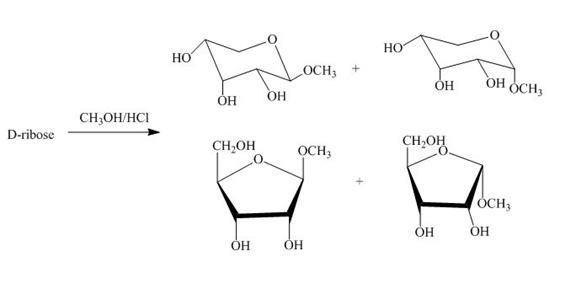
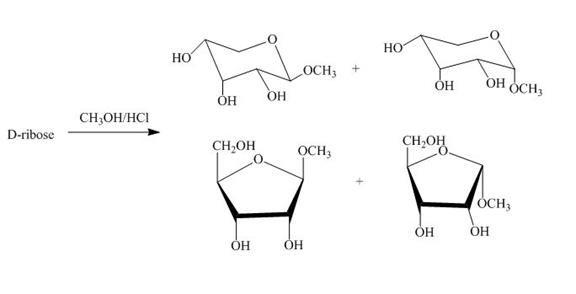
Figure 4
The D-ribose on reaction with methanol and hydrochloric acid is converted into the acetal. The acetal formed is found in both forms alpha and beta regardless of the configuration of D-ribose.
The product obtained when D-ribose is reacted with ![]() is shown in Figure 4.
is shown in Figure 4.
(e)
Interpretation:
The product obtained when the product of part (d) is reacted with ![]() (excess) and
(excess) and ![]() is to be stated.
is to be stated.
Concept introduction:
The methylation of the hydroxyl group of sugars is an important reaction. The methylation of hydroxyl groups is done with the help of methylating agent dimethyl sulfate in the presence of strong base sodium hydroxide.
Answer to Problem 24.35AP
The product obtained when the product of part (d) is reacted with ![]() (excess) and
(excess) and ![]() is shown below.
is shown below.
Explanation of Solution
The product obtained when the product of part (d) is reacted with ![]() (excess) and
(excess) and ![]() is shown below.
is shown below.

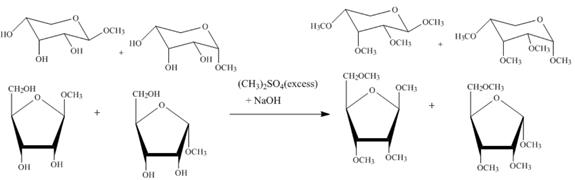
Figure 5
The four products of part (d) are alkylated in the strong base sodium hydroxide. The sodium hydroxide takes up the acidic proton of alcohol groups and converts them to alkoxide ion form. This alkoxide ion then attacks on the dimethyl sulfate (also a methylating agent) and take up the methyl group simultaneously eliminating the methyl sulfate group.
The product obtained when the product of part (d) is reacted with ![]() (excess) and
(excess) and ![]() is shown in Figure 5.
is shown in Figure 5.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry Study Guide and Solutions
- Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ?A Δ O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit ku F11arrow_forward१ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forwardDraw the molecules.arrow_forward
- Draw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward. Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forwardDraw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forward
- Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward[Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease draw.arrow_forward
- A chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forwardConsider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forward
