
(a)
Interpretation:
The name of the given compound has to be given.
Concept introduction:
According to
The order of priority is,
Depending on the number of carbon side chain of the amide, different types of amides can form.
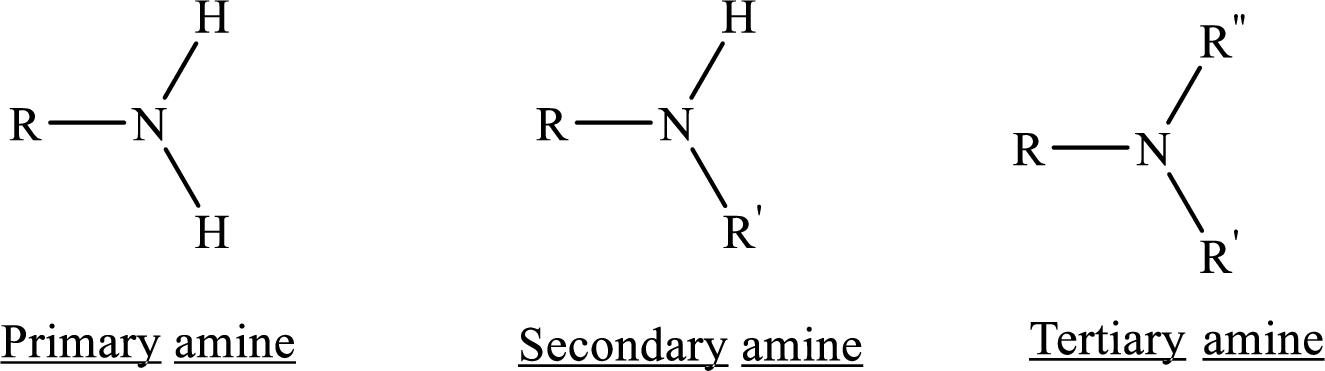
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
The IUPAC name for the carboxylic acid is written by replacing the ‘e’ of
If the compound contains amine and a functional group that has higher precedence than the amine group, then the amine group should be indicated with the prefix “amino”
R and S nomenclature: it is used to assign the molecule using CIP rules.
According to Cahn-Ingold-Prelog system,
The group attached to asymmetric center should be ranked based on the
Check the direction of arrow drawn in the direction of decreasing priority. If the arrow points clockwise direction, then the atom has R configuration. If the arrow points counterclockwise direction, then the atom has S configuration. If the group with lowest priority is not bonded by a hatched wedge, then interchange this group (lowest priority) by group bonded to hatched wedge and draw the arrow in priority order but the configuration is assigned as just reverse.
(b)
Interpretation:
The name of the given compound has to be given.
Concept introduction:
According to IUPAC nomenclature, the naming of compound is determined by the priority of the functional group if more than one functional group is present. The carbon attached to the functional group having most priority should get the least number while naming the compound.
The order of priority is,
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
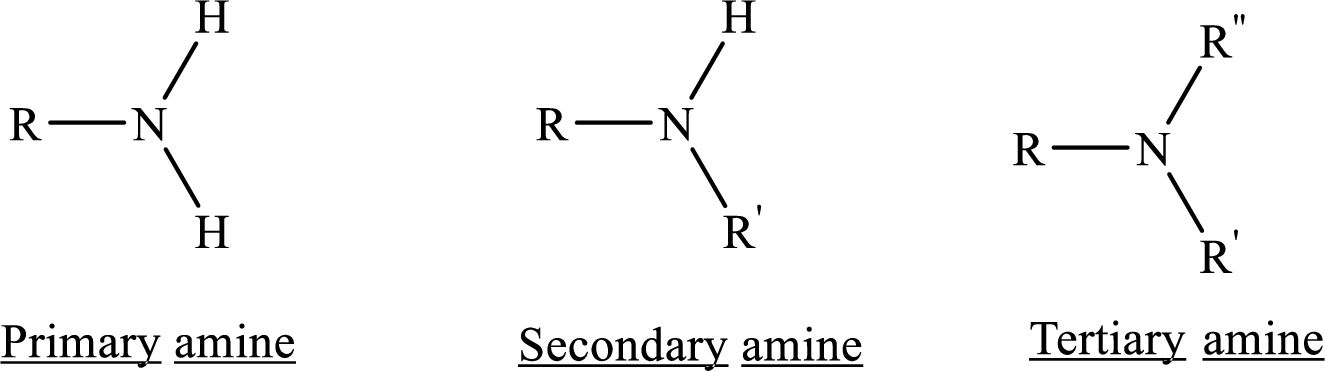
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Carboxylic acid: One
The IUPAC name for the carboxylic acid is written by replacing the ‘e’ of alkane to ‘oic acid’.
If the compound contains amine and a functional group that has higher precedence than the amine group, then the amine group should be indicated with the prefix “amino”
(c)
Interpretation:
The name of the given compound has to be given.
Concept introduction:
According to IUPAC nomenclature, the naming of compound is determined by the priority of the functional group if more than one functional group is present. The carbon attached to the functional group having most priority should get the least number while naming the compound.
The order of priority is,
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
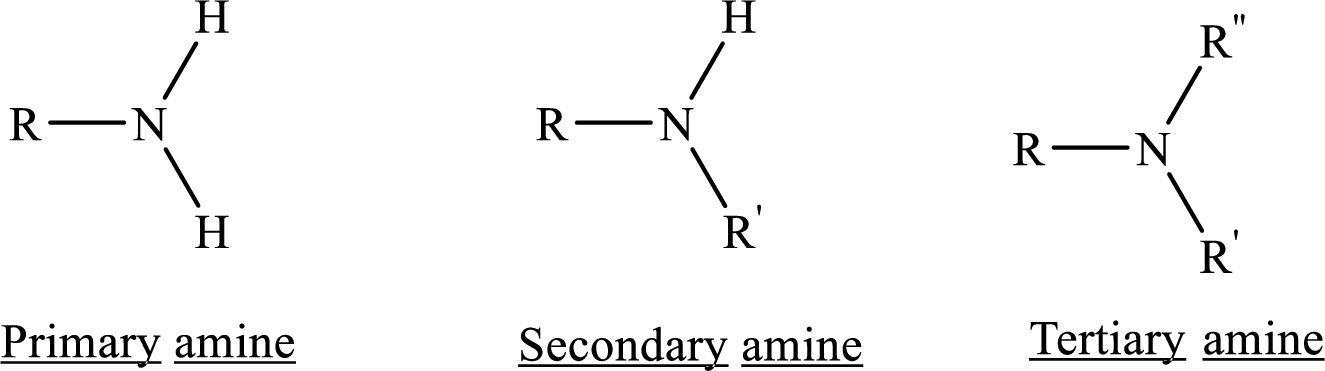
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Carboxylic acid: One
The IUPAC name for the carboxylic acid is written by replacing the ‘e’ of alkane to ‘oic acid’.
If the compound contains amine and a functional group that has higher precedence than the amine group, then the amine group should be indicated with the prefix “amino”
Common name of amine:
Alkyl groups attached to nitrogen atom of amine group must follow alphabetical order with the suffix “-amine”.
Trending nowThis is a popular solution!

Chapter 23 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Don't use ai to answer I will report you answerarrow_forwardConsider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forward
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

