
(a)
Interpretation:
Synthesis of 3-nitrophenol has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Conversion of
Aromatic amines converted to arenediazonium salt by reacting with
(b)
Interpretation:
Synthesis of 3-bromo nitrobenzene has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
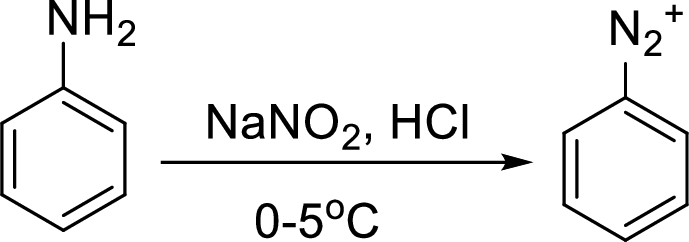
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
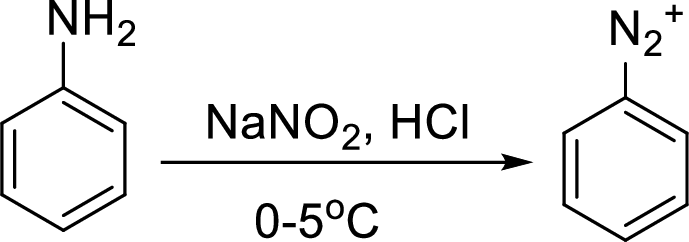
Sandmeyer reaction: It reaction type of organic reaction where the diazonium group in an arenediazonium salt gets replaced by
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
(c)
Interpretation:
Synthesis of 1,3-dihydroxybenzene has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
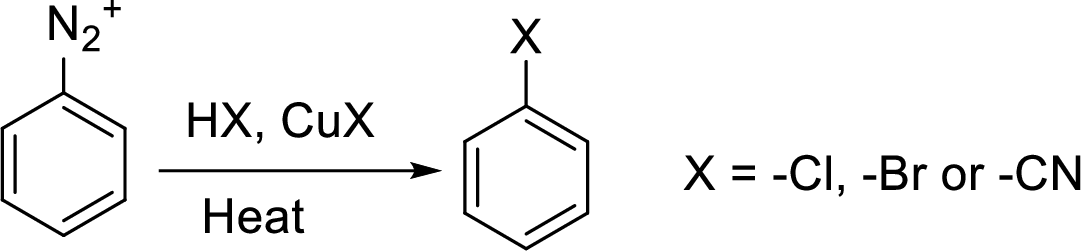
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
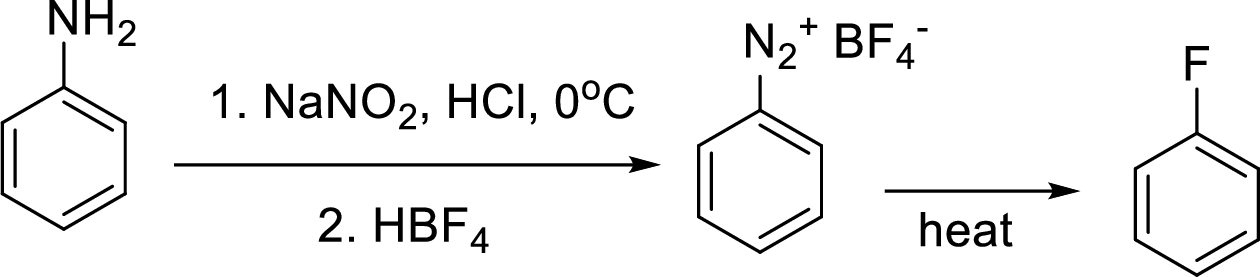
(d)
Interpretation:
Synthesis of 3-fluoroaniline has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Schiemann reaction: It is a method used to introduce fluorine into an aromatic ring. The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
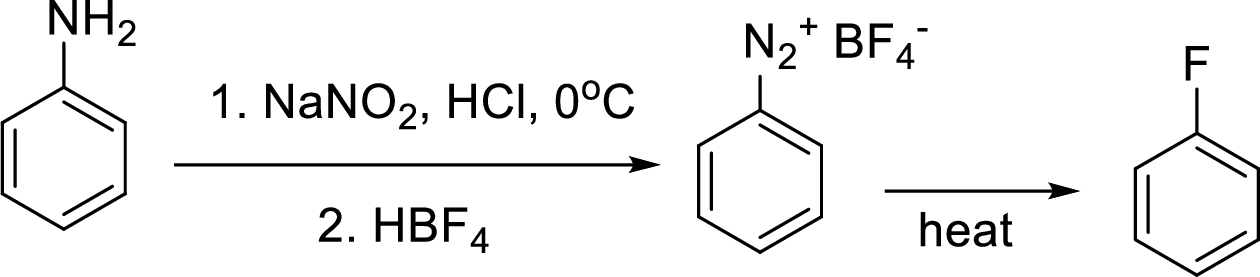
(e)
Interpretation:
Synthesis of 3-fluorophenol has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Conversion of aromatic amines to phenol:
Aromatic amines converted to arenediazonium salt by reacting with
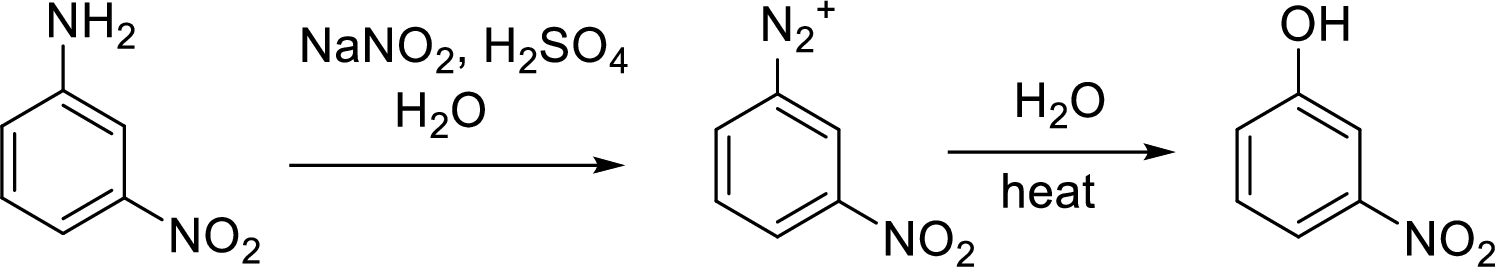
Schiemann reaction: It is a method used to introduce fluorine into an aromatic ring. The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
(f)
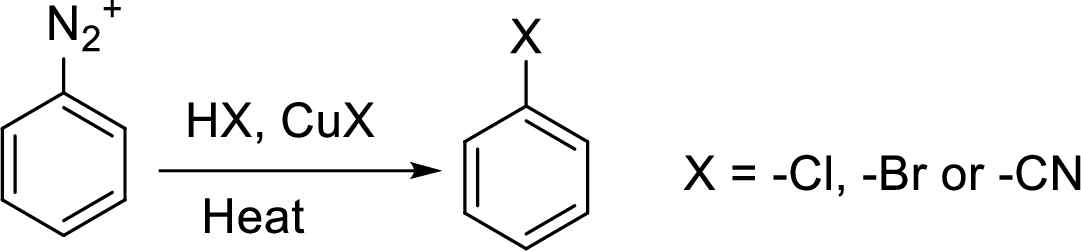
Interpretation:
Synthesis of 3-hydroxybenzonitrile has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
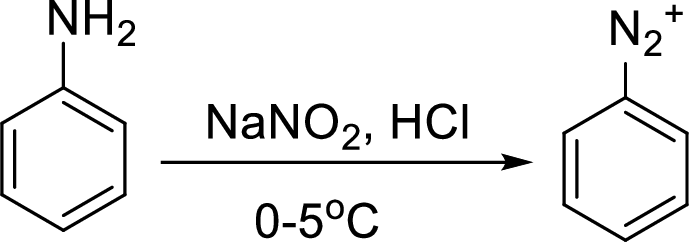
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
Sandmeyer reaction: It reaction type of organic reaction where the diazonium group in an arenediazonium salt gets replaced by
Trending nowThis is a popular solution!

Chapter 23 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Predict the major products of this reaction: ་ ་ + H NaOH ? Δ excess Note that the second reactant is used in excess, that is, there is much more of the second reactant than the first. If there won't be any products, just check the box under the drawing area instead.arrow_forwardP A student claims the right-hand side of the reaction in the drawing area below shows the product of a Claisen condensation. • If the student is correct, complete the reaction by adding the necessary organic reactants to the left-hand side, and by adding any necessary reagents and reaction conditions above and below the arrow. • If the student is incorrect, because it's not possible to obtain this product from a Claisen condensation, check the box under the drawing area instead. those that will minimize any byproducts or competing • Note for advanced students: If you have a choice, use the most efficient reactants and reagents reactions. - ☐ ☐ : ☐ + I Х Click and drag to start drawing a structure.arrow_forwardidentify the relationship between the structures and H- OH HO H H- OH and HO H H -ОН HO H Br and Brarrow_forward
- The right-hand side of this reaction shows the product of an aldol condensation. What are the reactants missing from the left-hand side? Draw them below. ? NaOH Δ If there aren't any reactants that would lead to these products under the reaction conditions given, just check the box under the drawing area. Note for advanced students: don't worry if the reactants you propose might also make some other products under these reaction conditions. Just make sure the product above is one of the major products.arrow_forwardPlease help! I need to identify four labeled unknown bottles based off of their colors doing titration using phenlphtalein. I've included my answers, but I wanted to make sure they were correct and if not, what will be correct thank you in advance.arrow_forwardAn organic chemistry Teaching Assistant (TA) suggested in your last discussion section that there is only one major organic product of the following reaction and that this reaction builds a ring. If the TA is right, draw the product in the drawing area below. If the TA is wrong, just check the box below the drawing area. NaOH ?arrow_forward
- A student suggests that the molecule on the right can be made from a single molecule that doesn't have a ring. If the student is correct, draw the starting material below, otherwise, check the box under the drawing area. Click and drag to start drawing a structure. : ☐ + NaOH टेarrow_forwardRate = k [I]1.7303[S2O82-]0.8502, Based on your rate, write down a mechanism consistent with your results and indicate which step is the rate determining step.arrow_forward36. Give the major product(s) of each of the following reactions. Aqueous work-up steps (when necessary) have been omitted. a. CH3CH=CHCH3 b. CH3CH2CH2CCH3 H,PO₂, H₂O, A (Hint: See Section 2-2.) 1. LIAIH. (CH,CH,),O 2. H', H₂O H NaBH, CH,CH₂OH d. Br LIAIH. (CH,CH,)₂O f. CH3 NaBH, CH,CH,OH (CH3)2CH H NaBH, CH,CH₂OH Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
