
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 31P
Draw the products of the following reaction, where T is Tritium:
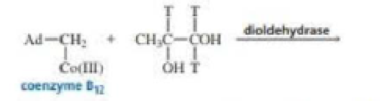
(Hint: Tritium is 3H, a hydrogen atom with two neutrons. Although a C — T bond breaks more slowly than a C — H bond, it is still the first bond in the substrate that breaks.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Pleasssssseeee solve this question in cheeemsirty, thankss sir
Show work. don't give Ai generated solution
Show work. Don't give Ai generated solution
Chapter 23 Solutions
Organic Chemistry (8th Edition)
Ch. 23.1 - Prob. 2PCh. 23.1 - Prob. 3PCh. 23.2 - How many conjugated double bonds are there in a....Ch. 23.2 - Instead of adding to the 4a position and...Ch. 23.2 - Prob. 7PCh. 23.3 - Prob. 8PCh. 23.3 - Acetolactate synthase is another TPP-requiring...Ch. 23.3 - Acetolactate synthase transfers the acyl group of...Ch. 23.3 - Prob. 12PCh. 23.5 - Which compound is more easily decarboxylated?
Ch. 23.5 - Prob. 14PCh. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - The enzyme that catalyzes the C C bond cleavage...Ch. 23.5 - Propose a mechanism for the ,-elimination reaction...Ch. 23.6 - Ethanolamine ammonia lyase, a coenzyme...Ch. 23.6 - Prob. 20PCh. 23.7 - How do the structure of tetrahydrofolate and...Ch. 23.7 - What is the source of the methyl group in...Ch. 23.8 - Thiols such as ethanethiol and propanethiol can be...Ch. 23 - How does the metal ion in carboxypeptidase A...Ch. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - For each of the following reactions, name both the...Ch. 23 - Prob. 27PCh. 23 - When transaminated, the three branched-chain amino...Ch. 23 - What acyl groups have we seen transferred by...Ch. 23 - Propose a mechanism for the following reaction:Ch. 23 - Draw the products of the following reaction, where...Ch. 23 - When UMP is dissolved in T2O, exchange of T for H...Ch. 23 - Dehydratase is a PLP-requiring enzyme that...Ch. 23 - In addition to the reaction mentioned in Section...Ch. 23 - PLP can catalyze both ,-elimination reactions...Ch. 23 - The glycine cleavage system is a group of four...Ch. 23 - Prob. 37PCh. 23 - FADH2 reduces , -unsaturated thioesters to...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A Give the IUPAC name and a common name for the following ether: CH3-CH2-O-CH2-CH2-CH3 Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Up Part B Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Uparrow_forwardFrenkel and Schottky are intrinsic or extrinsic defects, point or linear defects.arrow_forwardSelect the correct option:a) Frenkel and Schottky defects are linear crystal defects.b) Schottky defects involve atomic motions in a crystal lattice.c) Frenkel defects are vacancies in a crystal lattice.d) None of the above is correct.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License