
Concept explainers
(a)
Interpretation:
The hybridization and bond angles of carbon is bonded via four single bonds to adjacent atoms should be determined.
Concept introduction:
Carbon
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Example – Ethane.
Example –
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(a)
Answer to Problem 2PS
The carbon is
Explanation of Solution
Carbon is bonded via four single bonds to adjacent atoms. Carbon is bonded with four single four hydrogen atoms.
The Lewis structure as shown below.

Let’s write the carbon electronic configuration:
Here, the carbon is
(b)
Interpretation:
The hybridization and bond angle of carbon which is bonded via two single bonds and one pi bond should be determined.
Concept introduction:
Carbon atomic number 6. Electronic configuration -
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Example – Ethane.
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(b)
Answer to Problem 2PS
The carbon is
Explanation of Solution
Carbon is bonded via two single bonds and one pi bond.
Side overlapping of the two adjacent carbon atoms of orbitals form pi-bond.
The Lewis structure as shown below.
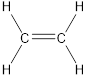
Let’s find the hybridization:
Here, the carbon is
(c)
Interpretation:
The hybridization and bond angles of carbon is bonded via one single bond and one triple bond should be determined.
Concept introduction:
Carbon atomic number 6. Electronic configuration -
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(c)
Answer to Problem 2PS
The carbon is
Explanation of Solution
Carbon is bonded via one single bond and one triple bond. Three pairs of electron are shared by two adjacent carbon atoms.
The Lewis structure as shown below.

Let’s find the Hybridization:
Here, the carbon is
(d)
Interpretation:
The hybridization and bond angles of carbon which are bonded via two double bonds should be determined.
Concept introduction:
Carbon atomic number 6. Electronic configuration -
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Example – Ethane.
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(d)
Answer to Problem 2PS
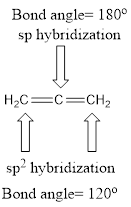
Explanation of Solution
Carbon is bonded via two double bonds. The three adjacent carbon atoms orbitals overlap to form two pi bonds.
The Lewis structure as shown below.
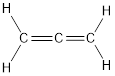
Let’s find the hybridization:
- (a) Hybridization of the terminal carbon atoms
Here, the carbon is
- (b) Hybridization of the central carbon atom
Here, the carbon is
Therefore,
The hybridization and bond angle is

Want to see more full solutions like this?
Chapter 23 Solutions
Owlv2 With Ebook, 1 Term (6 Months) Printed Access Card For Kotz/treichel/townsend/treichel's Chemistry & Chemical Reactivity, 10th
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H CH3NH3+ :0: :0: HO CH3NH2 HH iSelect to Add Arrows i Select to Add Arrows i HH CH3NH3+ CH3NH2 Select to Add Arrows i CH3NH3 CH3NH2 ايكدا HH Select to Add Arrowsarrow_forwardThe reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward
- 1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward20,0 Complete the electron pushing mechanism to y drawing the necomery unicaciones and carved on for Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation 458 Step 2: Added for the second step, inalment with), how the "counterion bar Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbondingarrow_forwardplease provide the structure for this problem, thank you!arrow_forward
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





