
(a)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
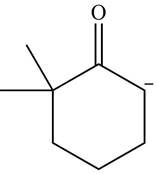
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
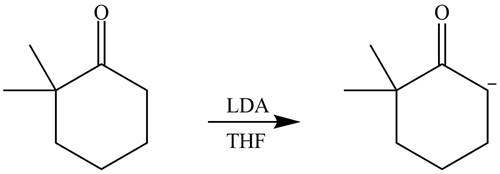
Figure 1
In this reaction,
(b)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
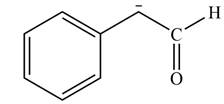
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
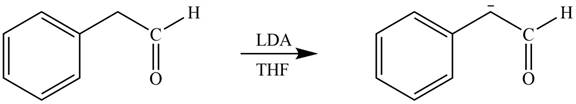
Figure 2
In this reaction,
(c)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
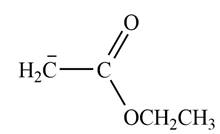
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
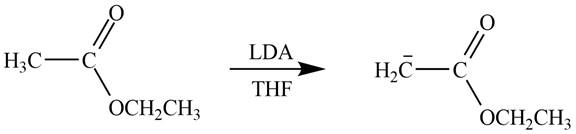
Figure 3
In this reaction, ethyl acetate is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom of the compound.
(d)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
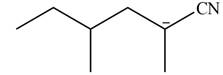
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
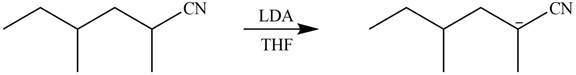
Figure 4
In this reaction,
(a) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
(b) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
(c) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
(d) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Want to see more full solutions like this?
Chapter 23 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Don't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





