
Concept explainers
(a)
Interpretation:
The sample that contains more monounsaturated fatty acids has to be identified.
Concept Introduction:
Monounsaturated fatty acid:
The compound contains only one carbon-carbon double bond in the fatty acid is called as monounsaturated fatty acid.
Example:

(b)
Interpretation:
The sample that contains more polyunsaturated fatty acids has to be identified.
Concept Introduction:
Polyunsaturated fatty acid:
The compound contains more than one carbon-carbon double bond in the fatty acid is called as polyunsaturated fatty acid.
Example:
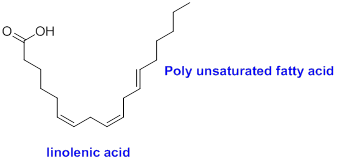
(c)
Interpretation:
The sample that contains fewer trans-fatty acids has to be identified.
Concept Introduction:
Trans-fats:
Trans-fats are the unsaturated fatty acid which occurs in very small amounts in nature, but can be prepared using hydrogenation reaction of vegetable oils.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- Obtain the sequence for the 5-HT receptor HTR1A and generate a hydropathy plot usingthe ExPASY tool ProtScale, the appropriate window, and the Kyte-Doolittle weightingalgorithm. How many transmembrane domains are present in this receptor? Attach yourhydropathy plot to your assignment.arrow_forwardCompare and contrast the structural features of the ion carrier valinomycin with those of achannel former like gramicidin. How does structural information help explain the mechanismby which these molecules conduct ions across membranes?arrow_forwardA typical integral membrane protein has a stretch (or stretches) of ~20 hydrophobic aminoacids that form an α-helix that spans the bilayer (as is found in membrane proteins such asglycophorin A and bacteriorhodopsin). Compare and contrast the molecular and structural features of gramicidin with a membrane-spanning α-helix. Explain how gramicidin can forman ion channel when a typical membrane-spanning α-helix cannot (eg, glycophorin A).arrow_forward
- The titration curve of alanine shows the ionization of two functional groups with pK values of 2.34 and 9.69, corresponding to the ionization of the carboxyl and the protonated amino groups, respectively. The titration of di-, tri-, and larger oligopeptides of alanine also shows the ionization of only two functional groups, although the experimental pK values are different. The table summarizes the trend in pK values. Amino acid or peptide Ala Ala-Ala pKj pk₂ 2.34 9.69 3.12 8.30 Ala-Ala-Ala 3.39 8.03 Ala-(Ala)-Ala, n ≥ 4 3.42 7.94 Modify the molecules to show the oligopeptide Ala-Ala-Ala. You can modify the molecules by moving, adding, deleting, or changing atoms, bonds, or charges. C Select c Draw Templates More H с N 0 S Cl H H | | || H CH3 H CH, H CH₂ Complete the statements about the the pK, values of the Ala-Ala-Ala oligopeptide. The pK₁ value of 3.39 is associated with the -COO group of Ala-Ala-Ala. The pK2 value of 8.03 is associated with the -NH group of Ala-Ala-Ala. Erase Q2 Q…arrow_forwardFacts from the bacterium mals and to dept kan apa in a peptide with antidic properties. This peptide complex with the call membrance of other hacterial species, leading in bacterial death The structure of the peptide has been determined from (a) Cmplete acid hydes of the peptide, followed by amino acid analys, yielded quiar anunt of Lan, Om, Pfx, Prxa, and Wall Cmtiti, an amino acid od prosentin pockets but present in some peptides. Com has the tracture H *H,N-CH-CH-CH, -C- COO (b) The weight of the peptide in approximately 1,200 Th (c) The peptide failed to undergo hydrolysis when treated with the Hydrolysis of the carbonyl-terminal residue of a polypeptide une "NH, the year. This call there Pro or the police does not contain a froz (d) Treatment of the peptide with 1-haw-2,4-dicherer (11N1), followed by complete hydrolysis and ched only from and the derivative NO, Н ON NHCHI CH, CH, C coo +NH, (Hint: The 2,4-diphenyl derivative involves the amino group of a side chain rather than the…arrow_forwardElectrophoresis Macmillan Learning Chymotrypsin is a protease with a molecular mass of 25.6 kDa. The figure shows a stained SDS polyacrylamide gel with a single band in lane I and three bands of lower molecular weight in lane 2. Lane I contains a preparation of chymotrypsin and lane 2 contains chymotrypsin pre-treated with performic acid. 1 2 Why does performic acid treatment of chymotrypsin generate three bands in lane 2? ° Chymotrypsin self-digests on the carboxyl-terminal side of phenylalanine, tryptophan, or tyrosine residues. The three peptides are impurities in the original chymotrypsin sample. Performic acid cleaves proteins on the carboxyl-terminal side of lysine and arginine residues. Performic acid cleaves the disulfide bonds holding together the three subunits of chymotrypsin. Correct Answerarrow_forward
- Extracts from the bacterium Bacillus brevis contain a peptide with antibiotic properties. This peptide forms complexes with metal ions and seems to disrupt ion transport across the cell membranes of other bacterial species, leading to bacterial death. The structure of the peptide has been determined from a series of observations. (a) Complete acid hydrolysis of the peptide, followed by amino acid analysis, yielded equimolar amounts of Leu, Orn, Phe, Pro, and Val. Orn is ornithine, an amino acid not present in proteins but present in some peptides. Orn has the structure H 'H,N-CH, - CH2 CH2 CH2 - C - COO- NH, (b) The molecular weight of the peptide is approximately 1,200 Da. (c) The peptide failed to undergo hydrolysis when treated with the enzyme carboxypeptidase. This enzyme catalyzes the hydrolysis of the carboxyl-terminal residue of a polypeptide unless the residue is Pro or the peptide does not contain a free carboxyl group. (d) Treatment of the intact peptide with…arrow_forwardAt a pH equal to the isoelectric point (pl) of alanine, the net charge of alanine is zero. Two structures can be drawn that have a net charge of zero, but the predominant form of alanine at its pl is zwitterionic. CH3 H,N CH3 ** H¸N-C H Zwitterionic H Uncharged OH Select statements that explain why alanine is predominantly zwitterionic at its pl. pk of alanine's amino group is more than its pl. pk of alanine's carboxyl group is more than its pl. PK of alanine's carboxyl group is less than its pl. pk of alanine's amino group is less than its pl. Correct Answer What fraction of alanine is in the completely uncharged form at its pl? 1 in 2.2 × 107 1 in 1.6 × 10² 1 in 4680 1 in 9460arrow_forwardHow does a voltage-gated sodium channel work? Specifically, how and why does a change in voltage trigger their opening? Please be detailedarrow_forward
- When sodium ions enter a neuron during depolarization, they trigger the opening of additional voltage-gated sodium channels nearby, creating a positive feedback loop where the influx of sodium ions further depolarizes the membrane, causing even more sodium channels to open and allowing more sodium ions to enter the cell, thus sustaining the depolarization process until the action potential peaks. But how and why exactly does the influx of sodium ions trigger more sodium channels to let in more sodium? Please explainarrow_forwardDraw the chemical structure of the tripeptide, HEL (L - amino acids), at pH = 7.0. Calculate isoelectric pointarrow_forwardCan someone draw what this would look like?arrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





