
(a)
Interpretation:
The isomer from the given pair that will undergo electrophilic
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
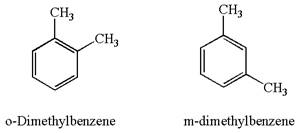
In both structures, benzene has two methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on the ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
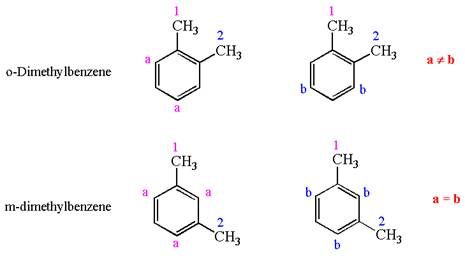
The activating positions of first methyl are indicated by letter
It is determined that
(b)
Interpretation:
The isomer from the given pair that will undergo electrophilic aromatic substitution faster is to be determined.
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
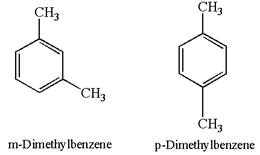
In both structures, the benzene has two methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
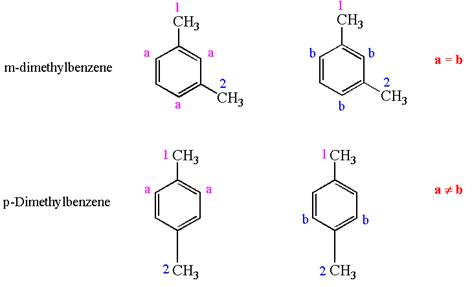
The activating positions of first methyl are indicated by letter
It is determined that
(c)
Interpretation:
The isomer from the given pair that will undergo electrophilic aromatic substitution faster is to be determined.
Concept introduction:
The electron donating groups activate whereas the electron withdrawing groups deactivate the aromatic ring. The electron donating groups are ortho-para directing, which means electrophilic aromatic substitution preferably occurs at the ortho-para position.
Answer to Problem 23.46P
The
Explanation of Solution
The given pair is
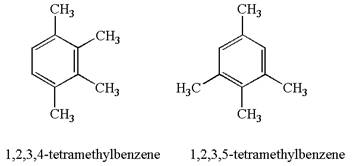
In both structures, the benzene has four methyl substituents, which are activating groups. They activate the ring at ortho-para direction. As the position of methyl groups is different in these structures, their activating position on ring would be different. In both the structures, each methyl activates the ring at two positions, as shown below:
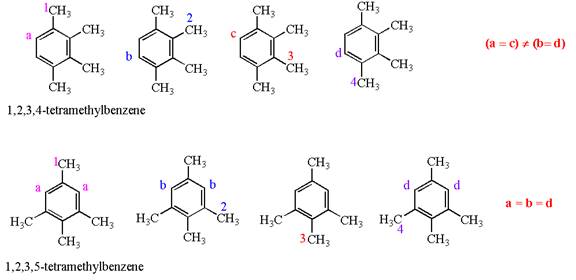
The activating positions of first methyl are indicated by letter
In case of
The methyl groups in
It is determined that
Want to see more full solutions like this?
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





