
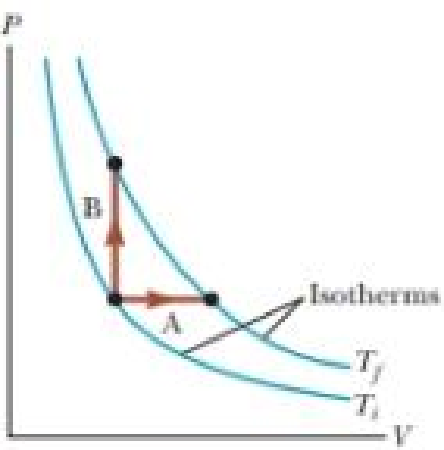
An ideal gas is taken from an initial temperature Ti to a higher final temperature Tf along two different reversible paths as shown in Figure 21.15. Path A is at constant pressure, and path B is at constant volume. What is the relation between the entropy changes of the gas for these paths? (a) ΔSA > ΔSB (b) ΔSA = ΔSB (c) ΔSA < ΔSB
Figure 21.15 (Quick Quiz 21.5) An ideal gas is taken from temperature Ti to Tf via two different paths.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Physics For Scientists And Engineers With Modern Physics, 9th Edition, The Ohio State University
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Campbell Essential Biology (7th Edition)
HUMAN ANATOMY
Cosmic Perspective Fundamentals
General, Organic, and Biological Chemistry - 4th edition
Genetics: From Genes to Genomes
- Statistical thermodynamics. The number of imaginary replicas of a system of N particlesa) cannot be greater than Avogadro's numberb) must always be greater than Avogadro's number.c) has no relation to Avogadro's number.arrow_forwardLab-Based Section Use the following information to answer the lab based scenario. A student performed an experiment in an attempt to determine the index of refraction of glass. The student used a laser and a protractor to measure a variety of angles of incidence and refraction through a semi-circular glass prism. The design of the experiment and the student's results are shown below. Angle of Incidence (°) Angle of Refraction (º) 20 11 30 19 40 26 50 31 60 36 70 38 2a) By hand (i.e., without using computer software), create a linear graph on graph paper using the student's data. Note: You will have to manipulate the data in order to achieve a linear function. 2b) Graphically determine the index of refraction of the semi-circular glass prism, rounding your answer to the nearest hundredth.arrow_forwardUse the following information to answer the next two questions. A laser is directed at a prism made of zircon (n = 1.92) at an incident angle of 35.0°, as shown in the diagram. 3a) Determine the critical angle of zircon. 35.0° 70° 55 55° 3b) Determine the angle of refraction when the laser beam leaves the prism.arrow_forward
- Use the following information to answer the next two questions. A laser is directed at a prism made of zircon (n = 1.92) at an incident angle of 35.0°, as shown in the diagram. 3a) Determine the critical angle of zircon. 35.0° 70° 55 55° 3b) Determine the angle of refraction when the laser beam leaves the prism.arrow_forwardNo chatgpt pls will upvotearrow_forwardA beam of alpha-particles of energy 7.3MeV is used.The protons emitted at an angle of zero degree are found to have energy of 9.34MeV.Find the Q-value of this reaction .arrow_forward
- An aluminum rod and a copper rod have the same length of 100cm at 5C. At what temperatures would one of the rods be 0.5 mm longer than the other? Which rod is longer at such temperature?arrow_forwardROTATIONAL DYNAMICS Question 01 A solid circular cylinder and a solid spherical ball of the same mass and radius are rolling together down the same inclined. Calculate the ratio of their kinetic energy. Assume pure rolling motion Question 02 A sphere and cylinder of the same mass and radius start from ret at the same point and more down the same plane inclined at 30° to the horizontal Which body gets the bottom first and what is its acceleration b) What angle of inclination of the plane is needed to give the slower body the same acceleration Question 03 i) Define the angular velocity of a rotating body and give its SI unit A car wheel has its angular velocity changing from 2rads to 30 rads seconds. If the radius of the wheel is 400mm. calculate ii) The angular acceleration iii) The tangential linear acceleration of a point on the rim of the wheel Question 04 in 20arrow_forwardQuestion B3 Consider the following FLRW spacetime: t2 ds² = -dt² + (dx² + dy²+ dz²), t2 where t is a constant. a) State whether this universe is spatially open, closed or flat. [2 marks] b) Determine the Hubble factor H(t), and represent it in a (roughly drawn) plot as a function of time t, starting at t = 0. [3 marks] c) Taking galaxy A to be located at (x, y, z) = (0,0,0), determine the proper distance to galaxy B located at (x, y, z) = (L, 0, 0). Determine the recessional velocity of galaxy B with respect to galaxy A. d) The Friedmann equations are 2 k 8πG а 4πG + a² (p+3p). 3 a 3 [5 marks] Use these equations to determine the energy density p(t) and the pressure p(t) for the FLRW spacetime specified at the top of the page. [5 marks] e) Given the result of question B3.d, state whether the FLRW universe in question is (i) radiation-dominated, (ii) matter-dominated, (iii) cosmological-constant-dominated, or (iv) none of the previous. Justify your answer. f) [5 marks] A conformally…arrow_forward
- SECTION B Answer ONLY TWO questions in Section B [Expect to use one single-sided A4 page for each Section-B sub question.] Question B1 Consider the line element where w is a constant. ds²=-dt²+e2wt dx², a) Determine the components of the metric and of the inverse metric. [2 marks] b) Determine the Christoffel symbols. [See the Appendix of this document.] [10 marks] c) Write down the geodesic equations. [5 marks] d) Show that e2wt it is a constant of geodesic motion. [4 marks] e) Solve the geodesic equations for null geodesics. [4 marks]arrow_forwardPage 2 SECTION A Answer ALL questions in Section A [Expect to use one single-sided A4 page for each Section-A sub question.] Question A1 SPA6308 (2024) Consider Minkowski spacetime in Cartesian coordinates th = (t, x, y, z), such that ds² = dt² + dx² + dy² + dz². (a) Consider the vector with components V" = (1,-1,0,0). Determine V and V. V. (b) Consider now the coordinate system x' (u, v, y, z) such that u =t-x, v=t+x. [2 marks] Write down the line element, the metric, the Christoffel symbols and the Riemann curvature tensor in the new coordinates. [See the Appendix of this document.] [5 marks] (c) Determine V", that is, write the object in question A1.a in the coordinate system x'. Verify explicitly that V. V is invariant under the coordinate transformation. Question A2 [5 marks] Suppose that A, is a covector field, and consider the object Fv=AAμ. (a) Show explicitly that F is a tensor, that is, show that it transforms appropriately under a coordinate transformation. [5 marks] (b)…arrow_forwardHow does boiling point of water decreases as the altitude increases?arrow_forward
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





