
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 78GQ
The glycinate ion, H2NCH2CO2−, formed by deprotonation of the amino acid glycine, can function as a bidentate ligand, coordinating to a metal through the nitrogen of the amino group and one of the oxygen atoms.
Glycinate ion, a bidentate ligand
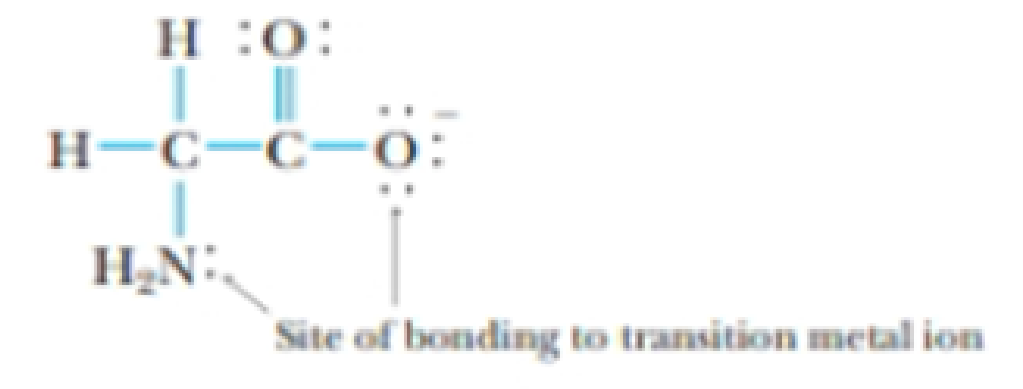
A copper complex of this ligand has the formula Cu(H2NCH2CO2)2(H2O)2. For this complex, determine the following:
(a) the oxidation state of copper
(b) the coordination number of copper
(c) the number of unpaired electrons
(d) whether the complex is diamagnetic or paramagnetic
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)
Bookmarks
Profiles Tab Window Help
Chemical Formula - Aktiv Che X
+
→ C
11
a
app.aktiv.com
Google Chrome isn't your default browser Set as default
Question 12 of 16
Q Fri Feb 2
Verify it's you
New Chrome availabl-
Write the balanced molecular chemical equation for the reaction in aqueous solution for
mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be
sure to include the proper phases for all species within the reaction.
3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq)
ean Ui
mate co
ence an
climate
bility inc
ulnerabili
women,
main critic
CLIMATE-INI
ernational
+
10
O
2
W
FEB
1
+
4-
3-
2-
2
2
(
3
4
NS
28
2
ty
56
+
2+
3+
4+
7
8
9 0
5
(s)
(1)
Ch
O
8
9
(g) (aq)
Hg
NR
CI
Cr
x H₂O
A
80
Q
A
DII
A
F2
F3
FA
F5
F6
F7
F8
F9
#3
EA
$
do 50
%
6
CO
&
7
E
R
T
Y
U
8
(
9
0
F10
34
F11
川
F12
Subr
+
delete
0
{
P
}
Deducing the reactants of a Diels-Alder reaction
n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one
step, by moderately heating the reactants?
?
Δ
• If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any
arrangement you like.
• If your answer is no, check the box under the drawing area instead.
Explanation Check
Click and drag to start drawing a structure.
>
Chapter 22 Solutions
Chemistry & Chemical Reactivity
Ch. 22.4 - (a) What is the formula of a complex ion composed...Ch. 22.4 - (a) Determine the metals oxidation number and...Ch. 22.4 - Name the following coordination compounds. (a)...Ch. 22.5 - What types of isomers are possible for the...Ch. 22.6 - Prob. 22.5CYUCh. 22.7 - Prob. 22.6CYUCh. 22.7 - Prob. 1.1ACPCh. 22.7 - Copper has a face-centered cubic unit cell. If...Ch. 22.7 - Prob. 1.3ACPCh. 22.7 - If a patient is given 10.0 mg of cisplatin, what...
Ch. 22.7 - Prob. 2.2ACPCh. 22.7 - How are the d electrons of Pt distributed in a...Ch. 22.7 - What are the electron configurations for Nd and...Ch. 22.7 - Prob. 3.2ACPCh. 22.7 - Prob. 3.3ACPCh. 22.7 - Prob. 3.4ACPCh. 22 - Identify, based on the position in the periodic...Ch. 22 - Prob. 2PSCh. 22 - Prob. 3PSCh. 22 - Prob. 4PSCh. 22 - Prob. 5PSCh. 22 - Iron is the most abundant transition element in...Ch. 22 - Prob. 7PSCh. 22 - Prob. 8PSCh. 22 - Prob. 9PSCh. 22 - Prob. 10PSCh. 22 - Identify a cation of a first series transition...Ch. 22 - Match up the isoelectronic ions on the following...Ch. 22 - The lanthanide contraction is given as an...Ch. 22 - Prob. 14PSCh. 22 - Prob. 15PSCh. 22 - Prob. 16PSCh. 22 - Prob. 17PSCh. 22 - Prob. 18PSCh. 22 - Which of the following ligands is expected to be...Ch. 22 - One of the following nitrogen compounds or ions is...Ch. 22 - Prob. 21PSCh. 22 - Prob. 22PSCh. 22 - Prob. 23PSCh. 22 - Prob. 24PSCh. 22 - Prob. 25PSCh. 22 - Prob. 26PSCh. 22 - Prob. 27PSCh. 22 - Prob. 28PSCh. 22 - Prob. 29PSCh. 22 - Prob. 30PSCh. 22 - Give the name or formula for each ion or compound,...Ch. 22 - Prob. 32PSCh. 22 - Prob. 33PSCh. 22 - Prob. 34PSCh. 22 - Prob. 35PSCh. 22 - Prob. 36PSCh. 22 - Prob. 37PSCh. 22 - Prob. 38PSCh. 22 - Prob. 39PSCh. 22 - Prob. 40PSCh. 22 - Prob. 41PSCh. 22 - Prob. 42PSCh. 22 - Prob. 43PSCh. 22 - Prob. 44PSCh. 22 - Prob. 45PSCh. 22 - Prob. 46PSCh. 22 - Prob. 47PSCh. 22 - Prob. 48PSCh. 22 - Prob. 49PSCh. 22 - Prob. 50PSCh. 22 - In water, the titanium(III) ion, [Ti(H2O)6]3+, has...Ch. 22 - Prob. 52PSCh. 22 - Prob. 53GQCh. 22 - Prob. 54GQCh. 22 - How many unpaired electrons are expected for...Ch. 22 - Prob. 56GQCh. 22 - Which of the following complex ions is (are)...Ch. 22 - Prob. 58GQCh. 22 - How many geometric isomers are possible for the...Ch. 22 - For a tetrahedral complex of a metal in the first...Ch. 22 - Prob. 61GQCh. 22 - Prob. 62GQCh. 22 - Prob. 63GQCh. 22 - A platinum-containing compound, known as Magnuss...Ch. 22 - Prob. 65GQCh. 22 - Prob. 66GQCh. 22 - Prob. 67GQCh. 22 - How many geometric isomers of the complex ion...Ch. 22 - Prob. 69GQCh. 22 - Prob. 70GQCh. 22 - Prob. 71GQCh. 22 - The square-planar complex Pt(en)Cl2 has chloride...Ch. 22 - The complex [Mn(H2O)6]2+ has five unpaired...Ch. 22 - Experiments show that K4[Cr(CN)6] is paramagnetic...Ch. 22 - Give a systematic name or the formula for the...Ch. 22 - When CrCI3 dissolves in water, three different...Ch. 22 - Prob. 77GQCh. 22 - The glycinate ion, H2NCH2CO2, formed by...Ch. 22 - Prob. 79GQCh. 22 - Nickel and palladium both form complexes of the...Ch. 22 - The transition metals form a class of compounds...Ch. 22 - Cerium, as noted in Applying Chemical Principles:...Ch. 22 - Prob. 84GQCh. 22 - Two different coordination compounds containing...Ch. 22 - Prob. 89SCQCh. 22 - Prob. 90SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forwardif the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forward
- Deducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forwardPredict the major products of the following organic reaction: Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Larrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY