
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 35P
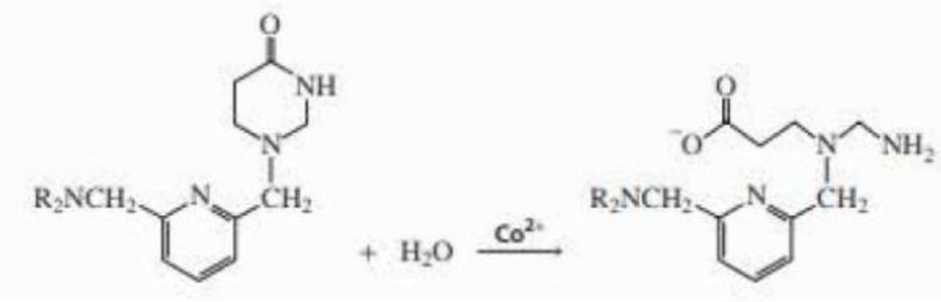
Co2+ catalyzes the hydrolysis of the lactam shown here. Propose a mechanism for the metal-ion catalyzed reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me with this I am very confused and I want to study.
The following three derivatives of succinimide are anticonvulsants and have found use in the treatment of epilepsy, particularly petit mal seizures.
Ph
Ph
`N'
`N'
ČH3
ČH3
Methsuximide
Ethosuximide
Phensuximide
Following is a synthesis of phensuximide.
CN
Ph
CN
Ph
CN
1. NaOH, H2O
2. HC, Н20
NaOEt
KCN
Ph-CHO
cOOEt
H
cOOEt
NC
COOEt
3. Нeat
Ethyl
cyanoacetate
(A)
(B)
Benzaldehyde
Ph
Ph
Ph
CH;NH2
НООС
СООН
Et0oC
COOEt
`N'
(C)
(D)
ČH3
Phensuximide
Methsuximide is formed by a similar pathway to that shown for phensuximide. Draw the structure of the compound that reacts with ethyl cyanoacetate in the synthesis of
methsuximide.
Rank the attached compounds in order of increasing basicity andexplain the order you chose.
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY
Ch. 22.2 - Compare each of the mechanisms listed here with...Ch. 22.2 - Prob. 3PCh. 22.2 - Prob. 4PCh. 22.3 - a. Draw the mechanism for the following reaction...Ch. 22.5 - Prob. 7PCh. 22.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 22.6 - Prob. 9PCh. 22.7 - Prob. 10PCh. 22.7 - Prob. 12PCh. 22.7 - Prob. 13P
Ch. 22.9 - Which of the following amino acid side chains can...Ch. 22.9 - Which of the following C-terminal peptide bonds is...Ch. 22.9 - Carboxypeptidase A has esterase activity as well...Ch. 22.10 - Arginine and lysine side chains fit into trypsins...Ch. 22.10 - Explain why serine proteases do not catalyze...Ch. 22.11 - If H2 18O is used in the hydrolysis reaction...Ch. 22.11 - Draw the pH-activity profile for an enzyme that...Ch. 22.12 - The pHactivity profile for glucose-6-phosphate...Ch. 22.12 - Prob. 23PCh. 22.13 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 22.13 - What advantage does the enzyme gain by forming an...Ch. 22.13 - Prob. 26PCh. 22.13 - Prob. 27PCh. 22.13 - Aldolase shows no activity if it is incubated with...Ch. 22 - Which of the following parameters would be...Ch. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Indicate the type of catalysis that is occurring...Ch. 22 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 22 - Prob. 34PCh. 22 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 22 - there are two kinds of aldolases. Class I...Ch. 22 - Prob. 37PCh. 22 - The hydrolysis of the ester shown here is...Ch. 22 - Prob. 39PCh. 22 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 22 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 22 - Proof that an imine was formed between aldolase...Ch. 22 - Prob. 43PCh. 22 - a. Explain why the alkyl halide shown here reacts...Ch. 22 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify one substrate and one reagent that could be combined to make the completed product.arrow_forwardNaturally occurring compounds called cyanogenic glycosides, such as lotaustralin, release hydrogen cyanide, HCN, when treated with aqueous acid. The reaction occurs by hydrolysis of the acetal linkage to form a cyanohydrin, which then expels HCN and gives a carbonyl compound. (a) Show the mechanism of the acetal hydrolysis and the structure of the cyanohydrin that results. (b) Propose a mechanism for the loss of HCN, and show the structure of the carbonyl compound that forms.arrow_forwardWhen cyclohexanone is heated in the presence of a large amount of acetone cyanohydrin and a small amount of base, cyclohexanone cyanohydrin and acetone are formed. Propose a mechanism.arrow_forward
- When an α-hydroxy amide is treated with Br2 in aqueous NaOH under Hofmann rearrangement conditions, loss of CO2 occurs and a chain-shortened aldehyde is formed. The mechanism involves the following steps: Base abstracts an acidic amide proton, yielding amide anion 1; The amide anion reacts with bromine in an α-substitution reaction to give N-bromoamide 2. Abstraction of the remaining amide proton by base gives a resonance-stabilized bromoamide anion 3; Rearrangement occurs to yield isocyanate 4; Water adds to the isocyanate to yield carbamic acid 5; Elimination of CO2 yields carbinolamine 6; Following proton transfer, expulsion of ammonia yields the final product aldehyde. Write out the mechanism and then draw the structures of bromoamide anion 3 and amide anion 1.arrow_forwardEthanolamine ammonia lyase, a coenzyme B12–requiring enzyme, catalyzes the following reaction. Propose a mechanism for this reaction.arrow_forwarda OH CH2CNHCH3 T CH3 d OH CHCHNHCH3 CH3 Ephedrine can be synthesized via reductive amination plus other necessary reactions. Predict the necessary reactants and reagents that will give the intermediate and final products shown.arrow_forward
- The mechanism for acidic hydrolysis of a nitrile resembles the basic hydrolysis, exceptthat the nitrile is first protonated, activating it toward attack by a weak nucleophile (water).Under acidic conditions, the proton transfer (tautomerism) involves protonation on nitrogen followed by deprotonation on oxygen. Propose a mechanism for the acid-catalyzedhydrolysis of benzonitrile to benzamide.arrow_forwardPlease answer this question correctly please.arrow_forwardPropose a synthesis for the antihistamine histapyrrodine. NH2 ÇOOH Do -NH Histapyrrodine Pyrrolidine Ethylene oxide Aniline Benzoic acidarrow_forward
- Draw the mechanism for the following reaction if it involves specific-base catalysis.arrow_forwardPropose a multi-step synthesis pathway to create the indicated products. Use the following substrates as your only source of carbon. Utilize the acetylide ion method to form the new carbon-carbon bond.arrow_forwardThe reagent diisobutylaluminum hydride (DIBALH) reduces esters to aldehydes. When nitriles are treated with DIBALH followed by mild acid hydrolysis, the product is also an aldehyde. Propose a mechanism for this reduction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License