
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 32P
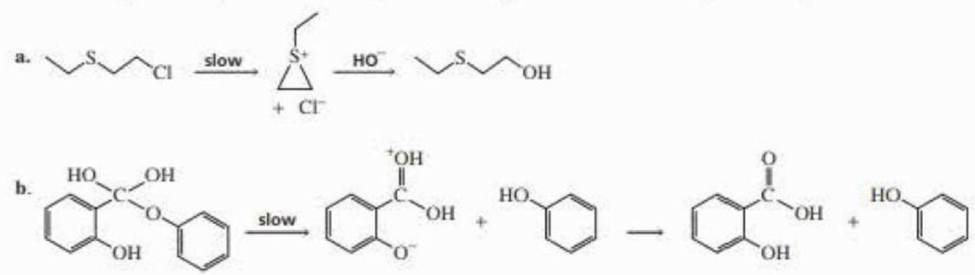
Indicate the type of catalysis that is occurring in the slow step in each of the following reaction sequences:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please correct answer and don't used hand raiting
Don't used hand raiting
Please correct answer and don't used hand raiting
Chapter 22 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 22.2 - Compare each of the mechanisms listed here with...Ch. 22.2 - Prob. 3PCh. 22.2 - Prob. 4PCh. 22.3 - a. Draw the mechanism for the following reaction...Ch. 22.5 - Prob. 7PCh. 22.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 22.6 - Prob. 9PCh. 22.7 - Prob. 10PCh. 22.7 - Prob. 12PCh. 22.7 - Prob. 13P
Ch. 22.9 - Which of the following amino acid side chains can...Ch. 22.9 - Which of the following C-terminal peptide bonds is...Ch. 22.9 - Carboxypeptidase A has esterase activity as well...Ch. 22.10 - Arginine and lysine side chains fit into trypsins...Ch. 22.10 - Explain why serine proteases do not catalyze...Ch. 22.11 - If H2 18O is used in the hydrolysis reaction...Ch. 22.11 - Draw the pH-activity profile for an enzyme that...Ch. 22.12 - The pHactivity profile for glucose-6-phosphate...Ch. 22.12 - Prob. 23PCh. 22.13 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 22.13 - What advantage does the enzyme gain by forming an...Ch. 22.13 - Prob. 26PCh. 22.13 - Prob. 27PCh. 22.13 - Aldolase shows no activity if it is incubated with...Ch. 22 - Which of the following parameters would be...Ch. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Indicate the type of catalysis that is occurring...Ch. 22 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 22 - Prob. 34PCh. 22 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 22 - there are two kinds of aldolases. Class I...Ch. 22 - Prob. 37PCh. 22 - The hydrolysis of the ester shown here is...Ch. 22 - Prob. 39PCh. 22 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 22 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 22 - Proof that an imine was formed between aldolase...Ch. 22 - Prob. 43PCh. 22 - a. Explain why the alkyl halide shown here reacts...Ch. 22 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Metal clusters and catalytic processes.arrow_forwardMetal clusters and catalysis.arrow_forwardQ1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License