
(a)
Interpretation:
The given reaction is to be completed.
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.88AP
The complete reaction is shown below.
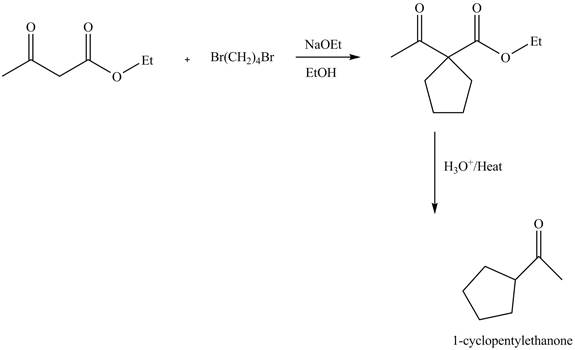
Explanation of Solution
The reaction of ethyl acetoacetate with dibromobutane in presence of base and ethanol results in the dialkylation reaction. It forms a cyclic product. This product undergoes hydrolysis reaction followed by decarboxylation reaction gives
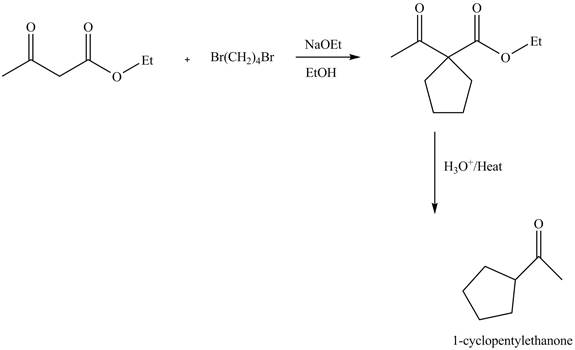
Figure 1
The complete reaction is shown in Figure 1.
(b)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Lactones are cyclic carboxylic esters analogous to one heteroatom cyclic ring. It is formed by intramolecular esterification reactions. Generally, five of six members lactones are more reactive. Lithium isopropylamine is a strong, non-nucleophilic base.
Answer to Problem 22.88AP
The complete reaction is shown below.
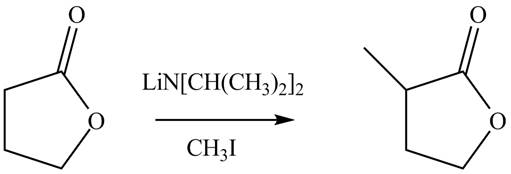
Explanation of Solution
The reaction of lactone which is a cyclic ester with lithium isopropylamide which is used as a base to abstract a proton. It forms an enolate ion which attacks a electrophile methyl iodide. It result in the formation of methyl substituted lactone compound. The given reaction is completed as shown below.
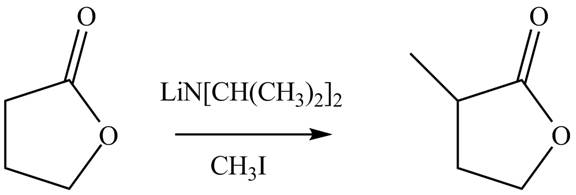
Figure 2
The complete reaction is shown Figure 2.
(c)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Aryl halides undergo substitution reactions only under drastic conditions. They generally are of two types addition-elimination and elimination reactions. The elimination addition reaction involves a benzyne intermediate. Whereas, in addition, an elimination reaction involves Meisenheimer complex formation.
Answer to Problem 22.88AP
The complete reaction is shown below.
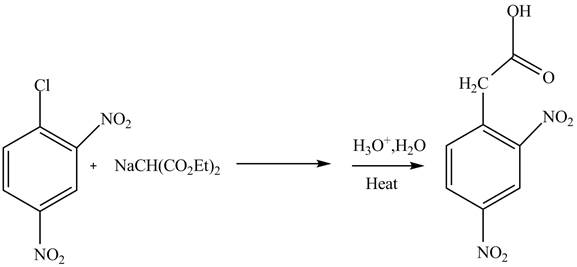
Explanation of Solution
The reaction of
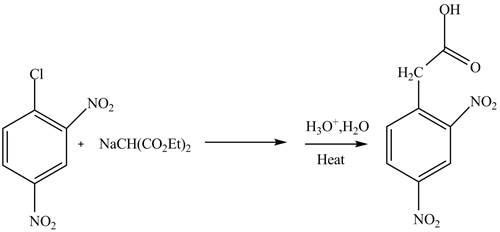
Figure 3
The complete reaction is shown in Figure 3.
(d)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The reduction of carbonyl compound is carried out by different reagents. For example
Answer to Problem 22.88AP
The complete reaction is shown below.
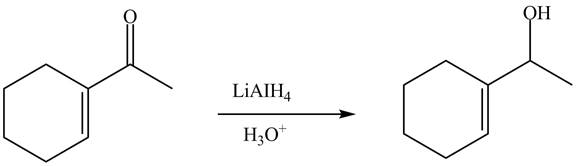
Explanation of Solution
The reaction of
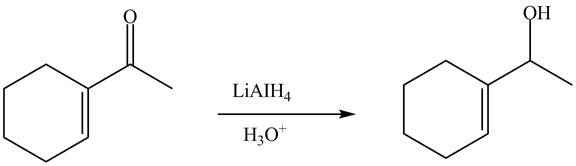
Figure 4
The complete reaction is shown in Figure 4.
(e)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Michael addition reaction is a nucleophilic addition reaction of an anion to
Answer to Problem 22.88AP
The complete reaction is shown below.
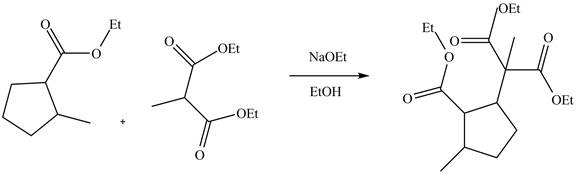
Explanation of Solution
The reaction of an ester with a base result in the abstraction of an acidic proton and form an enolate ion. This enolate ion undergoes

Figure 5
The complete reaction is shown in Figure 5.
(f)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The Knoevenagel condensation reaction is a modification of aldol condensation reaction. In this nucleophilic hydrogen atom adds to carbonyl group followed by dehydration reaction. The compound obtained is known as
Answer to Problem 22.88AP
The complete reaction is shown below.
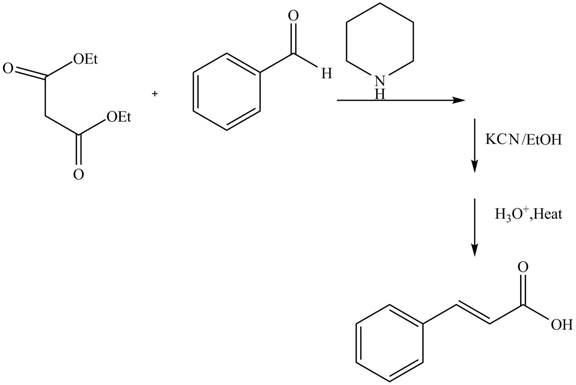
Explanation of Solution
The reaction of benzaldehyde and diethyl malonate in presence of piperdine base which forms an iminium intermediate ion. The reaction in presence of

Figure 6
The complete reaction is shown in Figure 6.
(g)
Interpretation:
The given reaction is to be completed.
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.88AP
The complete reaction is shown below.
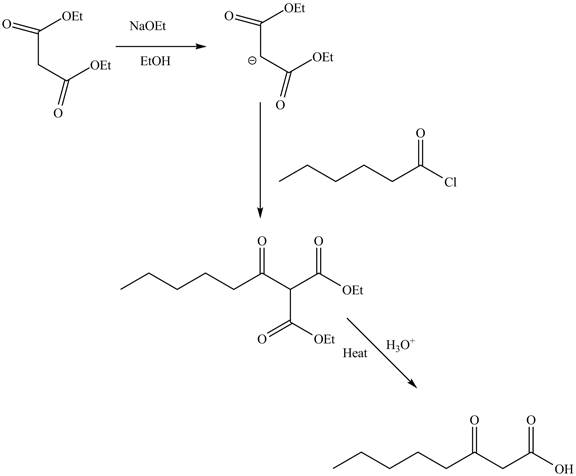
Explanation of Solution
The reaction of diethyl malonate in the presence of a base
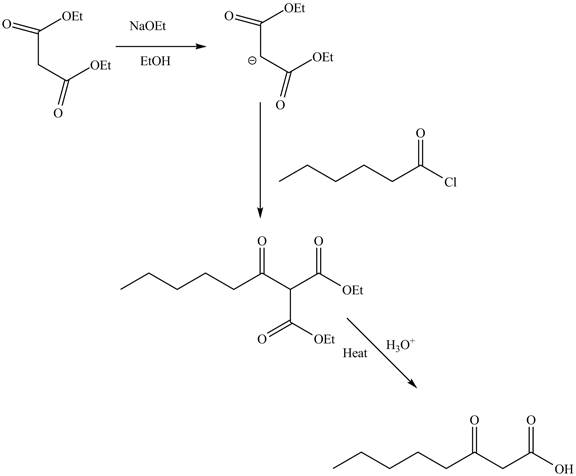
Figure 7
The complete reaction is shown in Figure 7.
(h)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Michael addition reaction is a nucleophilic addition reaction of an anion to
Answer to Problem 22.88AP
The complete reaction is shown below.
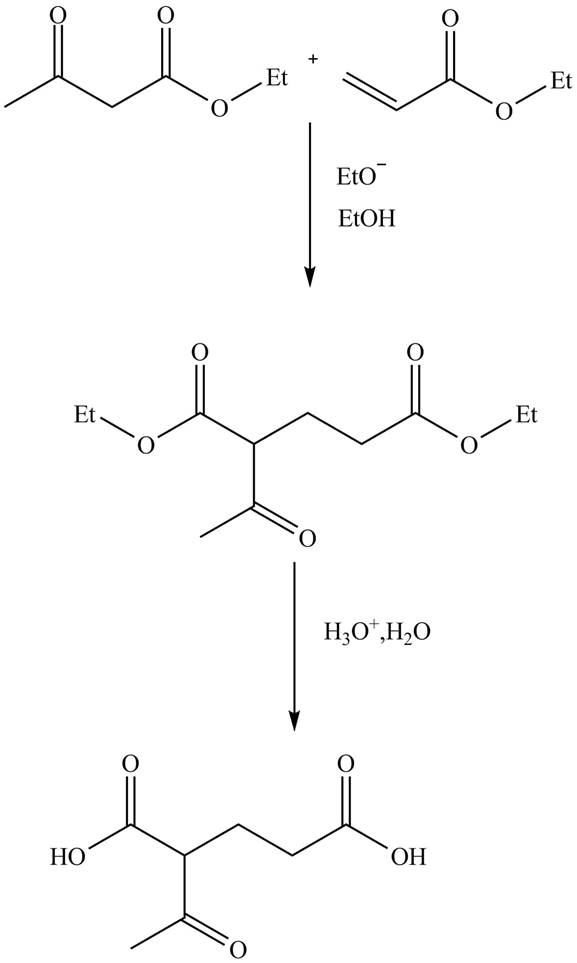
Explanation of Solution
The reaction of ethylacetoacetate with ethyl acrylate in presence of base
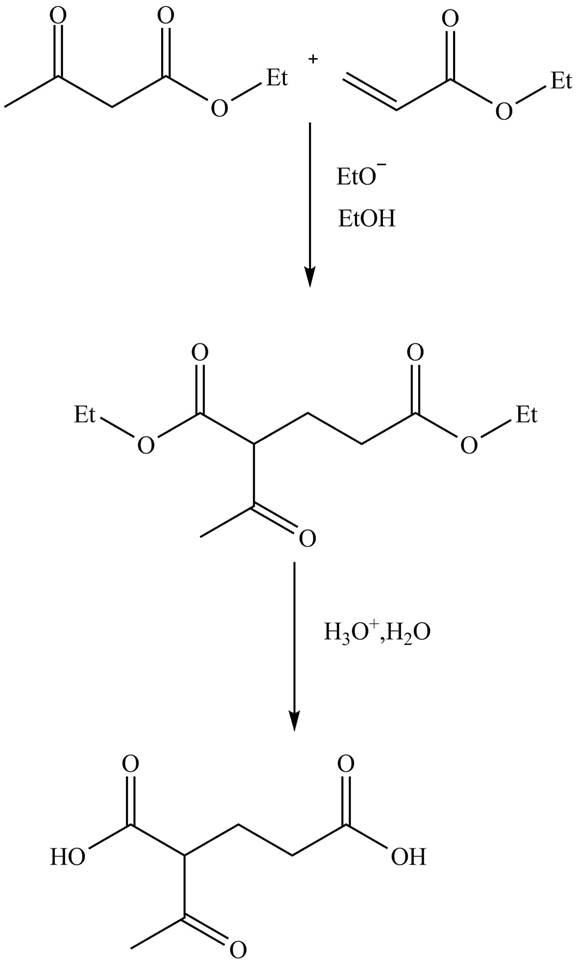
Figure 8
The complete reaction is shown in Figure 8.
(i)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Grignard reagents are
Answer to Problem 22.88AP
The complete reaction is shown below.
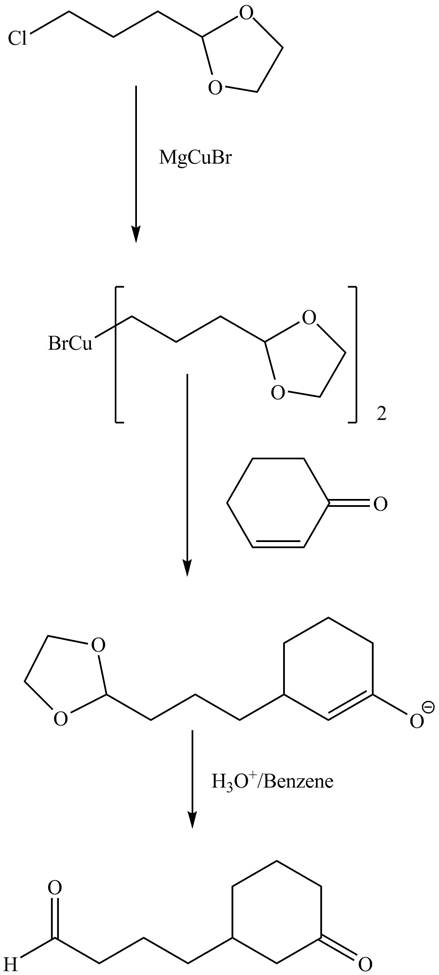
Explanation of Solution
The reaction of given dioxolane compound with
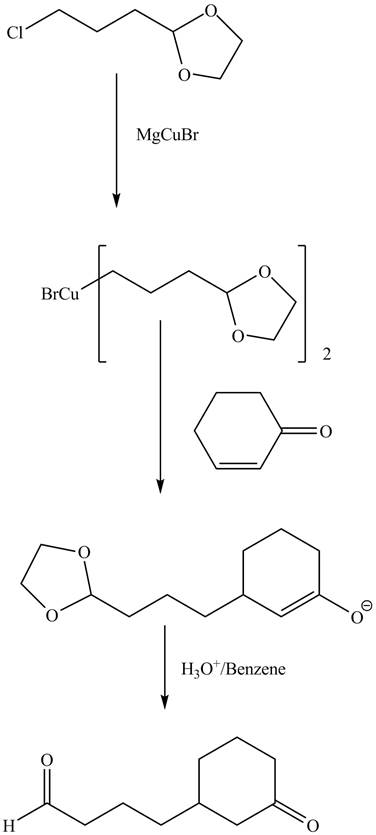
Figure 9
The complete reaction is shown in Figure 9.
(j)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases. Gilman reagent is a lithium and copper reagent. They react with organohalide to replace the halide group. They are used in Corey-House synthesis reaction.
Answer to Problem 22.88AP
The complete reaction is shown below.
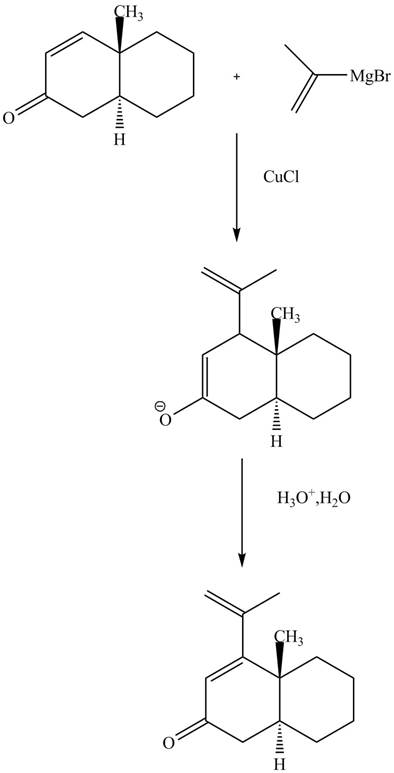
Explanation of Solution
The reaction of unsaturated Grignard reagent in the presence of
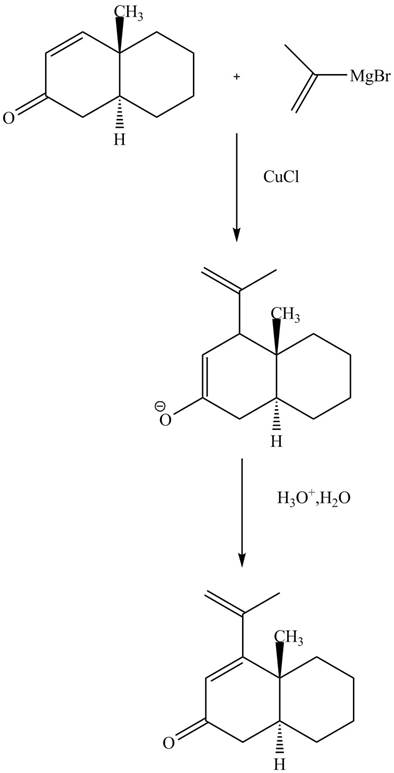
Figure 10
The complete reaction is shown in Figure 10.
(k)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases. Gilman reagent is a lithium and copper reagent. They react with organohalide to replace the halide group. They are used in Corey-House synthesis reaction.
Answer to Problem 22.88AP
The complete reaction is shown below.
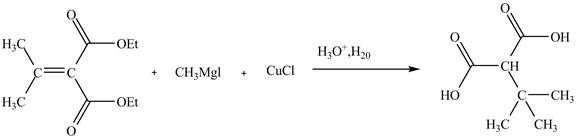
Explanation of Solution
The reaction of the Grignard reagent with
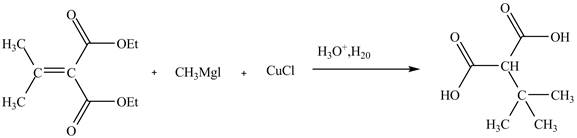
Figure 11
The complete reaction is shown in Figure 11.
(l)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an incoming electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring.
Answer to Problem 22.88AP
The complete reaction is shown below.
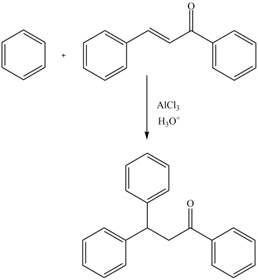
Explanation of Solution
The electrophilic substitution reaction of benzene with

Figure 12
The complete reaction is shown in Figure 12.
(m)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The Claisen condensation reaction in which two esters or one ester and a carbonyl compound react together to form
Answer to Problem 22.88AP
The complete reaction is shown below.
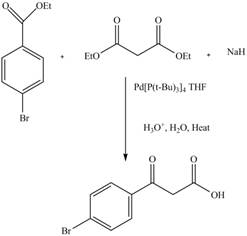
Explanation of Solution
The reaction of diethyl malonate in the presence a base forms an enolate ion. This attacked on another ester molecule and result in the formation of

Figure 13
The complete reaction is shown in Figure 13.
Want to see more full solutions like this?
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
