
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.12, Problem 21.33P
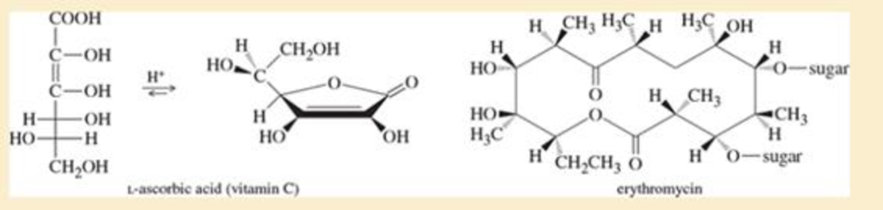
Problem 21-33
Propose a mechanism for the formation of 9-hydroxynonancwc acid lactone, as shown in the preceding figure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What are the reactions or reagents used? *see image
Provide the mechanism for this transformation: *see image
Assign all the signals individually (please assign the red, green and blue)
Chapter 21 Solutions
Organic Chemistry (9th Edition)
Ch. 21.2F - Name the following carboxylic acid derivatives,...Ch. 21.4A - Prob. 21.2PCh. 21.4A - Prob. 21.3PCh. 21.4A - Prob. 21.4PCh. 21.5C - Prob. 21.7PCh. 21.6 - When ethyl 4-hydroxybutyrate is heated in the...Ch. 21.6 - Propose a mechanism for the following ring-opening...Ch. 21.6 - Prob. 21.15PCh. 21.7B - Prob. 21.16PCh. 21.7C - Prob. 21.19P
Ch. 21.7C - Prob. 21.20PCh. 21.7C - Prob. 21.21PCh. 21.7D - Prob. 21.22PCh. 21.7D - The mechanism for acidic hydrolysis of a nitrile...Ch. 21.8A - Prob. 21.24PCh. 21.8C - Prob. 21.25PCh. 21.9 - Prob. 21.26PCh. 21.9 - Prob. 21.27PCh. 21.9 - Prob. 21.28PCh. 21.10 - Draw a mechanism for the acylation of anisole by...Ch. 21.10 - Prob. 21.30PCh. 21.11 - Prob. 21.31PCh. 21.11 - Prob. 21.32PCh. 21.12 - Problem 21-33 Propose a mechanism for the...Ch. 21.12 - Suggest the most appropriate reagent for each...Ch. 21.12 - Show how you would synthesize each compound,...Ch. 21.13 - Prob. 21.36PCh. 21.13 - Prob. 21.37PCh. 21.14 - Prob. 21.38PCh. 21.14 - Prob. 21.39PCh. 21.16 - Prob. 21.40PCh. 21.16 - Prob. 21.41PCh. 21 - Prob. 21.42SPCh. 21 - Give appropriate names for the following...Ch. 21 - Predict the major products formed when benzoyl...Ch. 21 - Predict the products of the following reactions....Ch. 21 - Prob. 21.46SPCh. 21 - Prob. 21.47SPCh. 21 - Prob. 21.48SPCh. 21 - Propose mechanisms for the following reactions.Ch. 21 - Prob. 21.51SPCh. 21 - An ether extraction of nutmeg gives large...Ch. 21 - Prob. 21.53SPCh. 21 - Show how you would accomplish the following...Ch. 21 - Prob. 21.55SPCh. 21 - Prob. 21.56SPCh. 21 - Prob. 21.57SPCh. 21 - Prob. 21.58SPCh. 21 - Prob. 21.59SPCh. 21 - Explain this curious result. What does this...Ch. 21 - Prob. 21.61SPCh. 21 - Prob. 21.62SPCh. 21 - Prob. 21.63SPCh. 21 - A chemist was called to an abandoned aspirin...Ch. 21 - Prob. 21.67SPCh. 21 - The IR spectrum, 13ONTVTR spectrum, and 1HNMR...Ch. 21 - Prob. 21.69SPCh. 21 - Prob. 21.70SPCh. 21 - Prob. 21.71SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward
- Can I please get help with this?arrow_forward1. Draw structures corresponding to each of the following names [3 ONLY]: A. 2,2,2-trichloroethanal (chloral). B. trans-3-isopropylcyclohexanecarbaldehyde C. What is the correct structure for 2-hydroxyacetophenone? Circle the letter of your response. a C 0 OH OH OH HO b. H3C CH 0 H d OH D. Provide IUPAC names for each structure below. 0 H C-H 0 0 CH3 H NO₂ E. The substance formed on addition of water to an aldehyde or ketone is called a hydrate or a/an: a. vicinal diol b. geminal diol C. acetal d. ketalarrow_forwardAssign this spectrumarrow_forward
- Redraw the tripeptide with or without its acidic hydrogensto demonstrate where the total charge of -2 comes from: *see imagearrow_forward2. Consider the data below to answer the following questions. Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. OH CH-COOH 0 HO CN C H30* C. H H HC N NaOH H₂O C=O 0 cyanohydrin H + NaCN + H₂Oarrow_forwardAssign all integrated peaksarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY