
(a)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(a)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is phenyl formate.
The molecular formula of phenyl formate is
The structure of phenyl formate is given as,
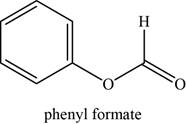
Figure 1
(b)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(b)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is cyclohexyl benzoate.
The molecular formula of cyclohexyl benzoate is
The structure of cyclohexyl benzoate is given as,
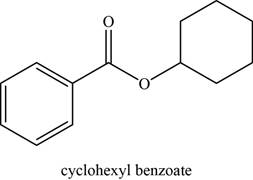
Figure 2
(c)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(c)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is cyclopentyl phenylacetate.
The molecular formula of cyclopentyl phenylacetate is
The structure of cyclopentyl phenylacetate is given as,
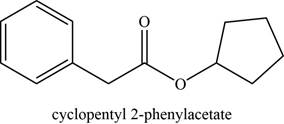
Figure 3
(d)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(d)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is N-butylacetamide.
The molecular formula of N-butylacetamide is
The structure of N-butylacetamide is given as,
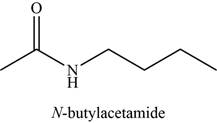
Figure 4
(e)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(e)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is N,N-dimethylformamide.
The molecular formula of N,N-dimethylformamide is
The structure of N,N-dimethylformamide is given as,
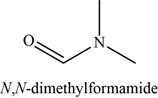
Figure 5
(f)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(f)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is benzoic propionic anhydride.
The molecular formula of benzoic propionic anhydride is
The structure of benzoic propionic anhydride is given as,
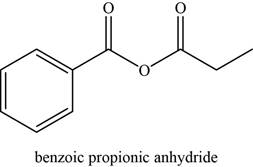
Figure 6
(g)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(g)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is benzamide.
The molecular formula of benzamide is
The structure of benzamide is given as,
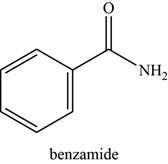
Figure 7
(h)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(h)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
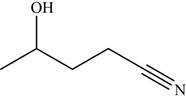
Figure 8
(i)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(i)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
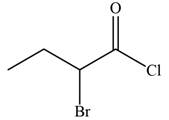
Figure 9
(j)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(j)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
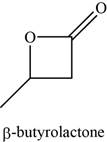
Figure 10
(k)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(k)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is phenyl isocyanate.
The molecular formula of phenyl isocyanate is
The structure of phenyl isocyanate is given as,
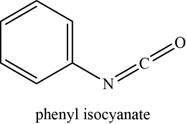
Figure 11
(l)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(l)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is cyclobutyl ethyl carbonate.
The molecular formula of cyclobutyl ethyl carbonate is
The structure of cyclobutyl ethyl carbonate is given as,
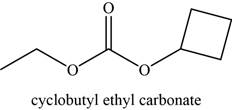
Figure 12
(m)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(m)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
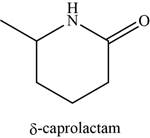
Figure 13
(n)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(n)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is trichloroacetic anhydride.
The molecular formula of trichloroacetic anhydride is
The structure of trichloroacetic anhydride is given as,
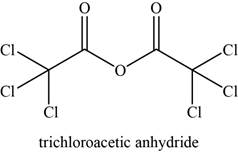
Figure 14
(o)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(o)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is Ethyl-N-methyl carbamate.
The molecular formula of Ethyl-N-methyl carbamate is
The structure of Ethyl-N-methyl carbamate is given as,
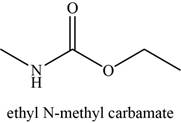
Figure 15
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry (9th Edition)
- in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forward
- calculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forward
- true or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forward
- the decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

