
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.88P
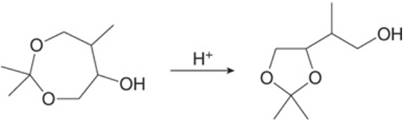
Draw a stepwise mechanism for the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The Concept of Aromaticity
21.15 State the number of 2p orbital electrons in each molecule or ion.
(a)
(b)
(e)
(f)
(c)
(d)
(h)
(i)
DA
(k)
21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the
Hückel criteria? Which, if planar, would be antiaromatic?
21.17 Which of the following structures are considered aromatic according to the Hückel
criteria?
---0-0
(a)
(b)
(c)
(d)
(e)
(h)
H
-H
.8.0-
21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a
heteroatom?
1. Show the steps necessary to make 2-methyl-4-nonene using a
Wittig reaction. Start with triphenylphosphine and an alkyl
halide. After that you may use any other organic or inorganic
reagents.
2. Write in the product of this reaction:
CH3
CH₂
(C6H5)₂CuLi
H₂O+
3. Name this compound properly, including stereochemistry.
H₂C
H3C
CH3
OH
4. Show the step(s) necessary to transform the compound on the
left into the acid on the right.
Bri
CH2
5. Write in the product of this
LiAlH4
Br
H₂C
OH
Chapter 21 Solutions
Organic Chemistry-Package(Custom)
Ch. 21 - Rank the following compounds in order of...Ch. 21 - Prob. 21.2PCh. 21 - Give the IUPAC name for each aldehyde.Ch. 21 - Prob. 21.4PCh. 21 - Give the IUPAC name for each ketone.Ch. 21 - Prob. 21.6PCh. 21 - Prob. 21.7PCh. 21 - The boiling point of is significantly higher than...Ch. 21 - Which carbonyl group in each pair absorbs at a...Ch. 21 - Problem 21.10 Draw the structure of all...
Ch. 21 - Prob. 21.11PCh. 21 - Prob. 21.12PCh. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Problem 21.15 Draw the product of each...Ch. 21 - Prob. 21.16PCh. 21 - Prob. 21.17PCh. 21 - Outline a synthesis of each Wittig reagent from...Ch. 21 - Draw the products including stereoisomers formed...Ch. 21 - Prob. 21.20PCh. 21 - Prob. 21.21PCh. 21 - Show two methods to synthesize each alkene: a...Ch. 21 - Problem 21.22 The product formed when reacts with...Ch. 21 - What 1 amine and carbonyl compound are needed to...Ch. 21 - Prob. 21.25PCh. 21 - Prob. 21.26PCh. 21 - Prob. 21.27PCh. 21 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.29 Draw the products of each...Ch. 21 - Problem 21.30 Label each compound as an acetal, a...Ch. 21 - Problem 21.31 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.32 Draw the products of each...Ch. 21 - Problem 21.33 Safrole is a naturally occurring...Ch. 21 - Problem 21.35 How would you use a protecting group...Ch. 21 - Prob. 21.35PCh. 21 - Problem 21.37 Two naturally occurring compounds...Ch. 21 - Problem 21.38 Draw the products of each...Ch. 21 - Prob. 21.38PCh. 21 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 21 - 21.41 Rank the following compounds in order of...Ch. 21 - Prob. 21.41PCh. 21 - Give the IUPAC name for each compound. a. d. g. b....Ch. 21 - Give the structure corresponding to each name. a....Ch. 21 - Including stereoisomers, draw the 11 aldehydes...Ch. 21 - Prob. 21.45PCh. 21 - Prob. 21.46PCh. 21 - What alkyl halide is needed to prepare each Wittig...Ch. 21 - 21.46 Draw the products of each reaction.
a. e....Ch. 21 - Prob. 21.49PCh. 21 - Prob. 21.50PCh. 21 - Prob. 21.51PCh. 21 - Prob. 21.52PCh. 21 - Prob. 21.53PCh. 21 - Rank the following compounds in order of...Ch. 21 - Which compound forms the higher concentration of...Ch. 21 - Prob. 21.56PCh. 21 - What Wittig reagent and carbonyl compound are...Ch. 21 - Prob. 21.58PCh. 21 - What reagents are needed to convert each compound...Ch. 21 - What reagents are needed to convert each compound...Ch. 21 - Prob. 21.61PCh. 21 - Devise a synthesis of each alkene using a Wittig...Ch. 21 - Devise a synthesis of each compound from...Ch. 21 - Prob. 21.64PCh. 21 - Prob. 21.65PCh. 21 - Prob. 21.66PCh. 21 - Prob. 21.67PCh. 21 - Prob. 21.68PCh. 21 - Prob. 21.69PCh. 21 - 21.64 Draw a stepwise mechanism for the following...Ch. 21 - 21.65 Draw a stepwise mechanism f or the following...Ch. 21 - Prob. 21.72PCh. 21 - 21.67 Draw a stepwise mechanism for each...Ch. 21 - 21.68 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.75PCh. 21 - Prob. 21.76PCh. 21 - Prob. 21.77PCh. 21 - Prob. 21.78PCh. 21 - Prob. 21.79PCh. 21 - 21.73 Although the carbonyl absorption of cyclic...Ch. 21 - 21.74 Use the and data to determine the...Ch. 21 - 21.75 A solution of acetone in ethanol in the...Ch. 21 - Compounds A and B have molecular formula ....Ch. 21 - 21.77 An unknown compound C of molecular formula ...Ch. 21 - 21.78 An unknown compound D exhibits a strong...Ch. 21 - Prob. 21.86PCh. 21 - -D-Glucose, a hemiacetal, can be converted to a...Ch. 21 - 21.81 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.89PCh. 21 - Prob. 21.90PCh. 21 - Prob. 21.91PCh. 21 - 21.86 Draw stepwise mechanism for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forward
- Potential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forward
- Draw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY