
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 21, Problem 21.79P
Interpretation Introduction
Interpretation:
The class of enzyme that catalyzes the following reaction should be determined:
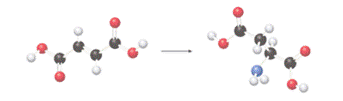
Concept introduction:
Enzymes are those biological molecules which increase the rate of a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series.
2) Calculate the ionization energy of He* and L2+ ions in their ground states.
3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.
Calculate the ionization energy of He+ and Li²+ ions in their ground states.
Thannnxxxxx sirrr
Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdva
Plleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AI
Chapter 21 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 21.2 - In addition to the amino and carboxyl groups, what...Ch. 21.2 - Draw both enantiomers of each amino acid in...Ch. 21.2 - Prob. 21.3PCh. 21.3 - Draw the structure of the amino acid valine at...Ch. 21.3 - Identify the amino acid shown with all uncharged...Ch. 21.4 - Identify the N-terminal and C-terminal amino acid...Ch. 21.4 - Prob. 21.7PCh. 21.4 - Prob. 21.8PCh. 21.4 - Prob. 21.9PCh. 21.4 - Prob. 21.10P
Ch. 21.4 - Prob. 21.11PCh. 21.5 - Prob. 21.12PCh. 21.6 - Prob. 21.13PCh. 21.6 - Prob. 21.14PCh. 21.6 - Prob. 21.15PCh. 21.7 - Why is hemoglobin more water soluble than ...Ch. 21.8 - Prob. 21.17PCh. 21.8 - Prob. 21.18PCh. 21.9 - Prob. 21.19PCh. 21.9 - Prob. 21.20PCh. 21.9 - Prob. 21.21PCh. 21.9 - Prob. 21.22PCh. 21.10 - Prob. 21.23PCh. 21.10 - Prob. 21.24PCh. 21.10 - Prob. 21.25PCh. 21.10 - Prob. 21.26PCh. 21.10 - Prob. 21.27PCh. 21.10 - Prob. 21.28PCh. 21.10 - Prob. 21.29PCh. 21.11 - Prob. 21.30PCh. 21 - The amino acid alanine is a solid at room...Ch. 21 - Why is phenylalanine water soluble but...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - Draw both enantiomers of each amino acid and label...Ch. 21 - Which of the following Fischer projections...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - (a) Identify the amino acid shown with all...Ch. 21 - Prob. 21.42PCh. 21 - Prob. 21.43PCh. 21 - Draw the structure of the neutral, positively...Ch. 21 - Prob. 21.45PCh. 21 - Prob. 21.46PCh. 21 - (a) Draw the structure of the two possible...Ch. 21 - Prob. 21.48PCh. 21 - Prob. 21.49PCh. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - Prob. 21.51PCh. 21 - For each tripeptide: [1] identify the amino acids...Ch. 21 - Prob. 21.53PCh. 21 - Prob. 21.54PCh. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Prob. 21.58PCh. 21 - Prob. 21.59PCh. 21 - Prob. 21.60PCh. 21 - Prob. 21.61PCh. 21 - Prob. 21.62PCh. 21 - What type of intermolecular forces exist between...Ch. 21 - What type of interaction occur at each of the...Ch. 21 - Prob. 21.65PCh. 21 - Draw the structures of the amino acids tyrosine...Ch. 21 - Prob. 21.67PCh. 21 - Prob. 21.68PCh. 21 - Prob. 21.69PCh. 21 - Prob. 21.70PCh. 21 - Prob. 21.71PCh. 21 - Hydrogen bonding stabilizes both the secondary and...Ch. 21 - Prob. 21.73PCh. 21 - Prob. 21.74PCh. 21 - Prob. 21.75PCh. 21 - Prob. 21.76PCh. 21 - What class of enzyme catalyzes each of the...Ch. 21 - What class of enzyme catalyzes each of the...Ch. 21 - Prob. 21.79PCh. 21 - Prob. 21.80PCh. 21 - Prob. 21.81PCh. 21 - What kind of reaction is catalyzed by each of the...Ch. 21 - Prob. 21.83PCh. 21 - How will each of the following changes affect the...Ch. 21 - Prob. 21.85PCh. 21 - Prob. 21.86PCh. 21 - Prob. 21.87PCh. 21 - Prob. 21.88PCh. 21 - Prob. 21.89PCh. 21 - Prob. 21.90PCh. 21 - Prob. 21.91PCh. 21 - Prob. 21.92PCh. 21 - Why must vegetarian diets be carefully balanced?Ch. 21 - Prob. 21.94PCh. 21 - Sometimes an incision is cauterized (burned) to...Ch. 21 - Why is insulin administered by injection instead...Ch. 21 - Prob. 21.97PCh. 21 - The silk produced by a silkworm is a protein with...Ch. 21 - Explain the difference in the mechanism of action...Ch. 21 - Prob. 21.100PCh. 21 - Prob. 21.101CPCh. 21 - Suggest a reason for the following observation....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY