
(a)
Interpretation:
The synthesis of given compound using a Claisen condensation is to be shown.
Concept introduction:
In Claisen condensation, ethyl ethanoate (ethyl acetate) is treated with sodium ethoxide, followed by acid work up, a
Answer to Problem 21.67P
The synthesis of given compound is shown below.
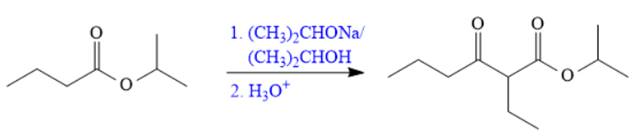
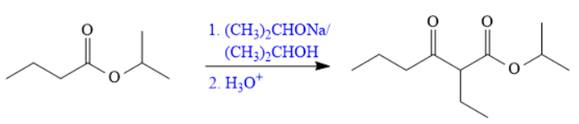
Explanation of Solution
The given compound is,
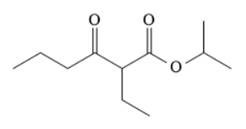
The disconnection of
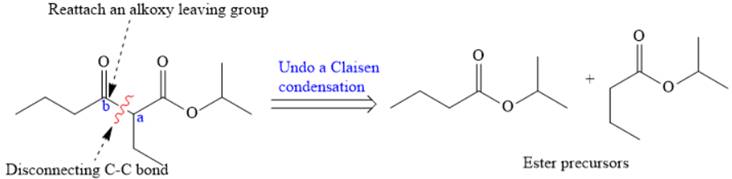
The Claisen reaction in the forward direction would proceed as follows:
The Claisen and Dieckmann condensations are new
(b)
Interpretation:
The synthesis of given compound using a Claisen condensation is to be shown.
Concept introduction:
In Claisen condensation, ethyl ethanoate (ethyl acetate) is treated with sodium ethoxide, followed by acid work up, a
Answer to Problem 21.67P
The synthesis of given compound is shown below.
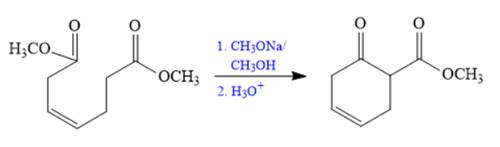
Explanation of Solution
The given compound is,
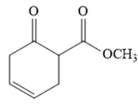
The disconnection of
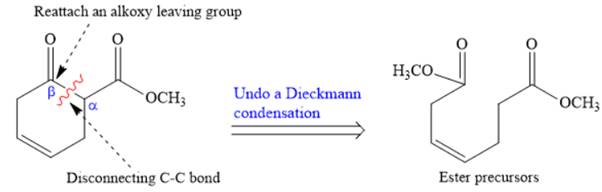
The Dieckmann reaction in the forward direction would proceed as follows:
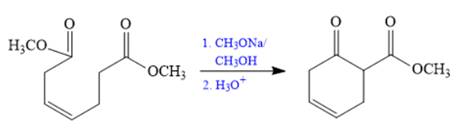
The Claisen and Dieckmann condensations are new
(c)
Interpretation:
The synthesis of given compound using a Claisen condensation is to be shown.
Concept introduction:
In Claisen condensation, ethyl ethanoate (ethyl acetate) is treated with sodium ethoxide, followed by acid work up, a
Answer to Problem 21.67P
The synthesis of given compound is shown below.
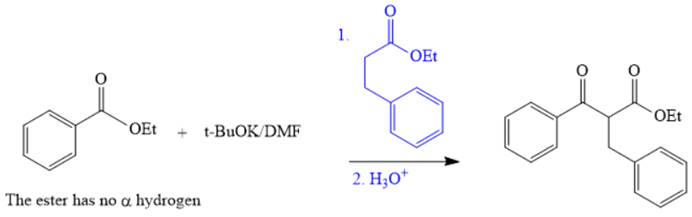
Explanation of Solution
The given compound is,
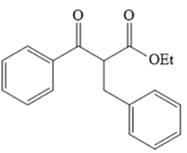
The disconnection of
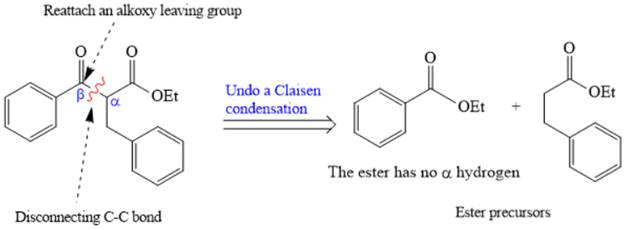
The crossed Claisen reaction in the forward direction would proceed as follows:
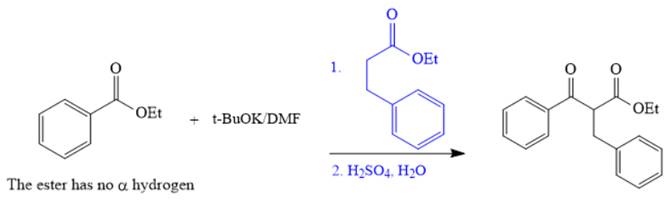
The Claisen and Dieckmann condensations are new
(d)
Interpretation:
The synthesis of given compound using a Claisen condensation is to be shown.
Concept introduction:
In Claisen condensation, ethyl ethanoate (ethyl acetate) is treated with sodium ethoxide, followed by acid work up, a
Answer to Problem 21.67P
The synthesis of given compound is shown below.
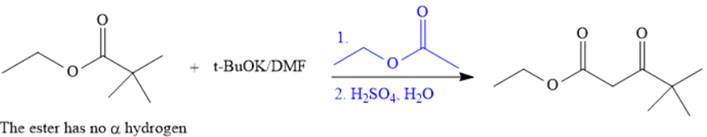
Explanation of Solution
The given compound is,
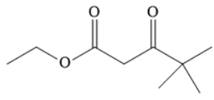
The disconnection of
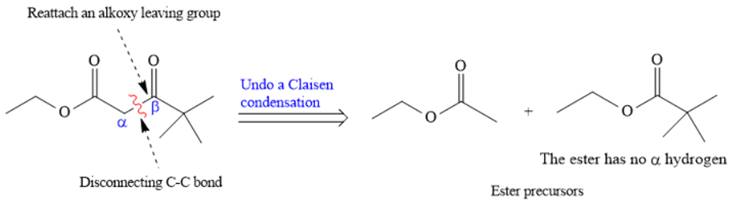
The crossed Claisen reaction in the forward direction would proceed as follows:
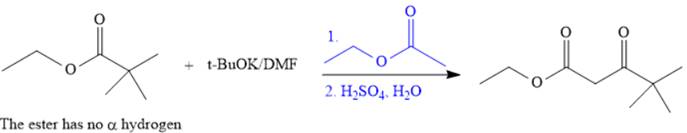
The Claisen and Dieckmann condensations are new
Want to see more full solutions like this?
Chapter 21 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- (c) (4pts) Mechanism: heat (E1) CH3OH + 1.5pts each _E1 _ (1pt) Br CH3OH (d) (4pts) Mechanism: SN1 (1pt) (e) (3pts) 1111 I H 10 Ill!! H LDA THF (solvent) Mechanism: E2 (1pt) NC (f) Bri!!!!! CH3 NaCN (3pts) acetone Mechanism: SN2 (1pt) (SN1) -OCH3 OCH3 1.5pts each 2pts for either product 1pt if incorrect stereochemistry H Br (g) “,、 (3pts) H CH3OH +21 Mechanism: SN2 (1pt) H CH3 2pts 1pt if incorrect stereochemistry H 2pts 1pt if incorrect stereochemistryarrow_forwardA mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixturearrow_forwardQ5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forward
- Calculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forwardWhere are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forward
- PLEASE HELP! URGENT!arrow_forwardWhere are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





