
Concept explainers
(a)
Interpretation: In the titration of glycine hydrochloride (1.0 M and 50 mL) and NaOH, the pH after the addition of 25.0 mL, 50.0 mL and 75.0 mL NaOH needs to be determined.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(a)
Explanation of Solution
Initially 1.0 M of 50 mL glycine hydrochloride is present. When 25 mL of NaOH is added to it, the following reaction takes place:
The pH of the solution can be calculated using the Henderson-Hesselbalch equation as follows:
Here,
Since, volume of the solution is same, number of moles can be used at the place of concentration.
Now, putting the values,
Thus, the pH when 25 mL of NaOH is added is 2.36.
Now, when 50 mL of NaOH is added the reaction can be represented as follows:
Now, the Zwitter ion can react with water as follows:
Now, total volume is 100 mL thus,
The ICE table can be represented as follows:
The acid dissociation constant can be represented as follows:
Here,
Thus,
Here, acid dissociation constant is very small thus, the value of x can be neglected when compared to 1. Thus,
Now,
Thus, the pH of the solution when 50 mL of NaOH is added is 5.04.
Now, when 75 mL of NaOH is added:
Further reaction takes place:
The pH can be calculated as follows:
Here,
Thus,
or,
Thus, the pH of the solution when 75 mL of NaOH is added is 9.77.
(b)
Interpretation: The titration curve needs to be drawn by indicating the major amino species present after addition of given volume of NaOH.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(b)
Explanation of Solution
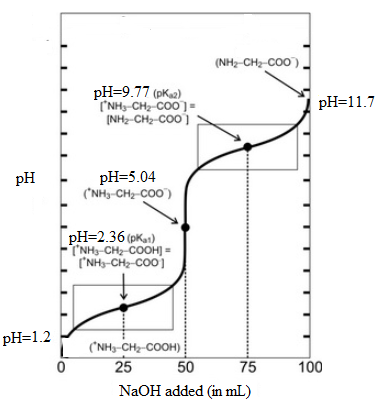
It is assumed that initially the pH is 1.2 and at second equivalent point is 11.7. Here, initial pH means when volume of NaOH is 0.0 mL and second equivalent point means when it is 100 mL. The values can be summarized as follows:
| Volume of NaOH added (mL) | pH |
| 0.0 | 1.2 |
| 25.0 | 2.36 |
| 50.0 | 5.04 |
| 75.0 | 9.77 |
| 100.0 | 11.7 |
Thus, the titration curve indicating the major amino species present at each point will be as follows:
(c)
Interpretation: The pH when the majority of amino acid molecules have net charge equal to zero needs to be determined.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(c)
Explanation of Solution
The titration curve is as follows:
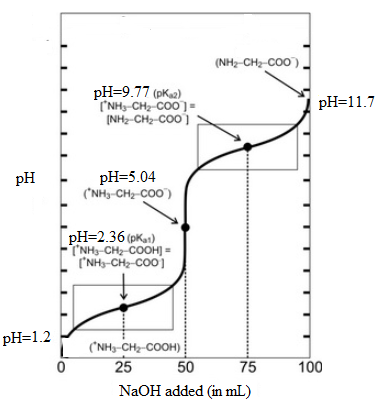
From the above titration curve, it can be seen that when 50 mL of NaOH is added, the majority of amino acid molecules have net charge equal to zero. At this volume corresponds to pH value 5.04. This is known as isoelectric point and denoted as pI and the point on graph corresponds to the equivalent point.
(d)
Interpretation: The pH when the net charge of the major amino acid species is +1/2 and -1/2 needs to be determined.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(d)
Explanation of Solution
The titration curve is as follows:
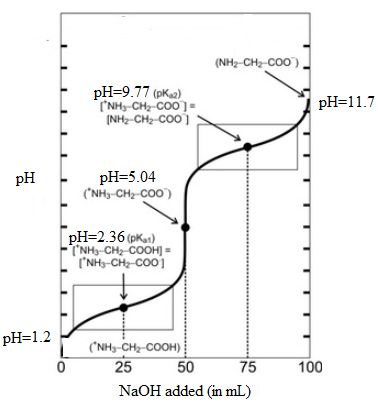
The point when the net charge of the major amino acid species is +1/2 corresponds to first half equivalent point. The volume of NaOH added at this point is 25 mL and corresponding pH is 2.36.
Similarly, the point when the net charge of the major amino acid species is -1/2 corresponds to second half equivalent point. The volume of NaOH added at this point is 75 mL and corresponding pH is 9.77.
Want to see more full solutions like this?
Chapter 21 Solutions
EBK CHEMICAL PRINCIPLES
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





