
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
4th Edition
ISBN: 9781260269284
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20, Problem 49P
Interpretation Introduction
(a)
Interpretation:
Monosaccharide formed after the hydrolysis of given disaccharides needs to be determined.
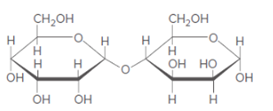
Concept introduction:
When disaccharides are hydrolyzed, a water molecule is added between the glyosidic bond that holds the monosaccharide together and results in the formation of respective monosaccharide.
Interpretation Introduction
(b)
Interpretation:
Monosaccharide formed after the hydrolysis of given disaccharides needs to be determined.
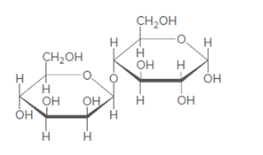
Concept introduction:
When disaccharides are hydrolyzed, a water molecule is added between the glyosidic bond that holds the monosaccharide together and results in the formation of respective monosaccharide.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?
If the concentration of A decreases exponentially
with time, what is the rate equation?
(A). -d[A]
(B).
dt
d[A]
= k[A]
e-kt
dt
Given the first-order reaction: aA → products. State its kinetic equation.
Chapter 20 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
Ch. 20.1 - Label the hemiacetal carbonthe carbon bonded to...Ch. 20.2 - Prob. 20.1PPCh. 20.2 - Prob. 20.2PCh. 20.2 - Prob. 20.3PCh. 20.2 - Prob. 20.4PCh. 20.2 - Prob. 20.5PCh. 20.2 - Prob. 20.2PPCh. 20.2 - Prob. 20.6PCh. 20.2 - Prob. 20.7PCh. 20.3 - Prob. 20.8P
Ch. 20.3 - Prob. 20.3PPCh. 20.3 - Prob. 20.4PPCh. 20.4 - Prob. 20.5PPCh. 20.4 - Prob. 20.6PPCh. 20.4 - Prob. 20.9PCh. 20.4 - Prob. 20.10PCh. 20.5 - Prob. 20.7PPCh. 20.5 - Prob. 20.8PPCh. 20.5 - Lactose contains both an acetal and a hemiacetal....Ch. 20.5 - Prob. 20.12PCh. 20.5 - Prob. 20.13PCh. 20.5 - Prob. 20.14PCh. 20.5 - Prob. 20.15PCh. 20.6 - Prob. 20.16PCh. 20.6 - Prob. 20.17PCh. 20.7 - Prob. 20.18PCh. 20.7 - Prob. 20.19PCh. 20.8 - Prob. 20.20PCh. 20 - Prob. 21PCh. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Prob. 25PCh. 20 - Prob. 26PCh. 20 - Prob. 27PCh. 20 - Prob. 28PCh. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Prob. 32PCh. 20 - Prob. 33PCh. 20 - Prob. 34PCh. 20 - Prob. 35PCh. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - Prob. 40PCh. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Prob. 43PCh. 20 - Prob. 44PCh. 20 - Prob. 45PCh. 20 - Prob. 46PCh. 20 - What product is formed when each compound is...Ch. 20 - What product is formed when each compound is...Ch. 20 - Prob. 49PCh. 20 - Prob. 50PCh. 20 - Prob. 51PCh. 20 - Prob. 52PCh. 20 - Prob. 53PCh. 20 - Prob. 54PCh. 20 - Prob. 55PCh. 20 - Prob. 56PCh. 20 - Prob. 57PCh. 20 - Prob. 58PCh. 20 - Prob. 59PCh. 20 - Prob. 60PCh. 20 - Prob. 61PCh. 20 - Prob. 62PCh. 20 - Prob. 63PCh. 20 - Prob. 64PCh. 20 - Prob. 65PCh. 20 - Prob. 66PCh. 20 - Prob. 67PCh. 20 - Prob. 68PCh. 20 - Prob. 69PCh. 20 - Prob. 70PCh. 20 - Prob. 71PCh. 20 - Prob. 72PCh. 20 - Prob. 73PCh. 20 - Prob. 74PCh. 20 - Prob. 75PCh. 20 - Prob. 76PCh. 20 - Prob. 77PCh. 20 - Prob. 78PCh. 20 - Prob. 79CPCh. 20 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
- How many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forwardWhich of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning