
Physics for Science and Engineering With Modern Physics, VI - Student Study Guide
4th Edition
ISBN: 9780132273244
Author: Doug Giancoli
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 44P
(II) An ideal gas of n moles undergoes the reversible process ab shown in the PV diagram of Fig. 20–20. The temperature Τ of the gas is the same at points a and b. Determine the change in entropy of the gas due to this process.
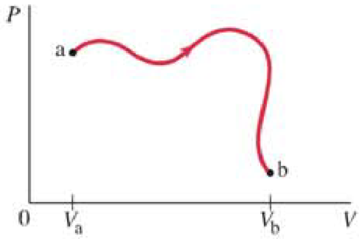
FIGURE 20–20
Problem 44.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
. (I) What is the change in entropy of 1.00 m3 of water at 0°C when it is frozen to ice at 0°C?
(i) Define the term entropy in thermodynamics.
(ii) In what way entropy is related to third law of thermodynamics?
Compare and contrast adiabatic processes and Cyclical processes in thermodynamics with necessary explanations and sketches.
Let 1.00 kg of liquid water at 100°C be converted to steam at a pressure of 1.01 * 105 Pa. The volume of water changes from an initial value of 1.00 * 10-3 m3 as a liquid to 1.671 m3 as steam. The heat of vaporization is 2256 kJ/kg.
(i) How much work is done by the system during the process?
(ii) How much energy is transferred as heat?
(iii) What is the change in the system’s energy during the process?
(2) Try to draw the following processes of ideal gas passing through point 1
on the given parameter p-v and T-s coordinate maps: constant pressure
process (n=0), constant temperature process (n=1), constant entropy
process (n=K) and constant volume process (n=∞). Mark accordingly
on the diagram.
Р
T +
1
V
S
Chapter 20 Solutions
Physics for Science and Engineering With Modern Physics, VI - Student Study Guide
Ch. 20.2 - An adiabatic process is defined as one in which no...Ch. 20.3 - A motor is running with an intake temperature TH =...Ch. 20.6 - A 1.00.kg piece of ice at 0C melts very slowly to...Ch. 20.9 - Prob. 1EECh. 20 - Prob. 1QCh. 20 - Can you warm a kitchen in winter by leaving the...Ch. 20 - Would a definition of heat engine efficiency as e...Ch. 20 - What plays the role of high-temperature and...Ch. 20 - Which will give the greater improvement in the...Ch. 20 - The oceans contain a tremendous amount of thermal...
Ch. 20 - Discuss the factors that keep real engines from...Ch. 20 - Prob. 8QCh. 20 - Describe a process in nature that is nearly...Ch. 20 - (a) Describe how heat could be added to a system...Ch. 20 - Suppose a gas expands to twice its original volume...Ch. 20 - Give three examples, other than those mentioned in...Ch. 20 - Which do you think has the greater entropy, 1 kg...Ch. 20 - (a) What happens if you remove the lid of a bottle...Ch. 20 - Prob. 15QCh. 20 - Prob. 16QCh. 20 - Prob. 17QCh. 20 - The first law of thermodynamics is sometimes...Ch. 20 - Powdered milk is very slowly (quasistatically)...Ch. 20 - Two identical systems are taken from state a to...Ch. 20 - It can he said that the total change in entropy...Ch. 20 - Use arguments, other than the principle of entropy...Ch. 20 - (I) A heat engine exhausts 7800 J of heat while...Ch. 20 - (I) A certain power plant puts out 580 MW of...Ch. 20 - (II) A typical compact car experiences a total...Ch. 20 - (II) A four-cylinder gasoline engine has an...Ch. 20 - (II) The burning of gasoline in a car releases...Ch. 20 - (II) Figure 2017 is a PV diagram for a reversible...Ch. 20 - (III) The operation of a diesel engine can be...Ch. 20 - (I) What is the maximum efficiency of a heat...Ch. 20 - (I) It is not necessary that a heat engines hot...Ch. 20 - (II) A heal engine exhausts its heat at 340C and...Ch. 20 - (II) (a) Show that the work done by a Carnot...Ch. 20 - (II) A Carnot engines operating temperatures are...Ch. 20 - (II) A nuclear power plant operates at 65% of its...Ch. 20 - (II) A Carnot engine performs work at the rate of...Ch. 20 - (II) Assume that a 65 kg hiker needs 4.0 103 kcal...Ch. 20 - (II) A particular car does work at the rate of...Ch. 20 - (II) A heat engine utilizes a heat source at 580C...Ch. 20 - (II) The working substance of a certain Carnot...Ch. 20 - (III) A Carnot cycle, shown in Fig. 20-7, has the...Ch. 20 - (III) One mole of monatomic gas undergoes a Carnot...Ch. 20 - (III) In an engine that approximates the Otto...Ch. 20 - (I) If an ideal refrigerator keeps its contents at...Ch. 20 - (I) The low temperature of a freezer cooling coil...Ch. 20 - (II) An ideal (Carnot) engine has an efficiency of...Ch. 20 - (II) An ideal heal pump is used to maintain the...Ch. 20 - (II) A restaurant refrigerator has a coefficient...Ch. 20 - (II) A heat pump is used to keep a house warm at...Ch. 20 - (II) (a) Given that the coefficient of performance...Ch. 20 - (II) A Carnot refrigerator (reverse of a Carnot...Ch. 20 - (II) A central heat pump updating as an air...Ch. 20 - (II) What volume of water at 0C can a freezer make...Ch. 20 - (I) What is the change in entropy of 250g of steam...Ch. 20 - (I) A 7.5-kg box having an initial speed of 4.0m/s...Ch. 20 - (I) What is the change in entropy of 1.00 m3 of...Ch. 20 - (II) If 1.00m3 of water at 0C is frozen and cooled...Ch. 20 - (II) If 0.45kg f water at 100C is changed by a...Ch. 20 - (II) An aluminum rod conducts 9.50 cal/s from a...Ch. 20 - (II) A 2.8-kg piece of aluminum at 43.0C is placed...Ch. 20 - (II) An ideal gas expands isothermally (T = 410 K)...Ch. 20 - (II) When 2.0 kg of water at 12.0C is mixed with...Ch. 20 - (II) (a) An ice cube of mass m at 0C is placed in...Ch. 20 - (II) The temperature of 2.0mol of an ideal...Ch. 20 - (II) Calculate the change in entropy of 1.00kg of...Ch. 20 - (II) An ideal gas of n moles undergoes the...Ch. 20 - (II) Two samples of an ideal gas are initially at...Ch. 20 - (II) A 150-g insulated aluminum cup at 15C is...Ch. 20 - (II) (a) Why would you expect the total entropy...Ch. 20 - (II) 1.00 mole of nitrogen (N2) gas and 1.00 mole...Ch. 20 - (II) Thermodynamic processes are sometimes...Ch. 20 - (III) The specific heat per mole of potassium at...Ch. 20 - (III) Consider an ideal gas of n moles with molar...Ch. 20 - (III) A general theorem states that the amount of...Ch. 20 - (III) Determine the work available in a 3.5-kg...Ch. 20 - (I) Use Eq. 2014 to determine the entropy of each...Ch. 20 - (II) Suppose that you repeatedly shake six coins...Ch. 20 - (II) Calculate the relative probabilities, when...Ch. 20 - (II) (a) Suppose you have four coins, all with...Ch. 20 - Prob. 58PCh. 20 - (II) Energy may be stored for use during peak...Ch. 20 - (II) Solar cells (Fig. 20-22) can produce about...Ch. 20 - Prob. 61PCh. 20 - It has been suggested that a heat engine could be...Ch. 20 - A heat engine takes a diatomic gas around the...Ch. 20 - A 126.5-g insulated aluminum cup at 18.00C is...Ch. 20 - (a) At a steam power plant, steam engines work in...Ch. 20 - (II) Refrigeration units can be rated in tons. A...Ch. 20 - Prob. 67GPCh. 20 - (a) What is the coefficient of performance of an...Ch. 20 - The operation of a certain heat engine takes an...Ch. 20 - A car engine whose output power is 155 hp operates...Ch. 20 - Suppose a power plant delivers energy at 850 MW...Ch. 20 - 1.00 mole of an ideal monatomic gas at STP first...Ch. 20 - Two 1100-kg cars are traveling 75 km/h in opposite...Ch. 20 - Metabolizing 1.0 kg of fat results in about 3.7 ...Ch. 20 - A cooling unit for a new freezer has an inner...Ch. 20 - Prob. 76GPCh. 20 - The Stirling cycle shown in Fig 20-27, is useful...Ch. 20 - A gas turbine operates under the Brayton cycle,...Ch. 20 - Thermodynamic processes can be represented not...Ch. 20 - An aluminum can, with negligible heat capacity, is...Ch. 20 - Prob. 81GPCh. 20 - A bowl contains a large number of red, orange, and...
Additional Science Textbook Solutions
Find more solutions based on key concepts
3. CAUTION Why is genetic drift aptly named?
a. It causes allele frequencies to drift up or down randomly.
b. I...
Biological Science (6th Edition)
60. You are 9.0 m from the door of your bus, behind the bus, when it pulls away with an acceleration of 1.0 m/...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Distinguish between microevolution, speciation, and macroevolution.
Campbell Essential Biology (7th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
10. Suppose you are holding a box in front of you and away from your body by squeezing the sides, as shown in F...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- In an isochoric process, heat is added to 10 mol of monoatomic ideal gas whose temperature increases from 273 to 373 K. What is the entropy change of the gas?arrow_forwardDoes the entropy increase for a Carnot engine for each cycle?arrow_forwardIs it possible for a system to have an entropy change if it neither absorbs nor emits heat during a reversible? transition? What happens it the process is irreversible?arrow_forward
- For the Carnot cycle of Figure 4.12, what is the entropy change of the hot reservoir, the cold reservoir, and the universe? Figure 4.11 The four processes of the Carnot cycle. The working substance is assumed to be an ideal gas whose thermodynamic path MNOP is represented in Figure 4.12. Figure 4.12 The total work done by the gas in the Carnot cycle is shown and given by the area enclosed by the loop MNOPM.arrow_forwardSuppose 20 g of ice at 0 is added to 300 g of water at 60 . What is the total change in entropy of the mixture after it reaches thermal equilibrium?arrow_forwardA cylinder containing three moles of a monatomic ideal gas is heated at a constant pressure of 2 atm. The temperature of the gas changes from 300 K to 350 K as a result of the expansion. Find work done (a) on the gas; and (b) by the gas.arrow_forward
- A glass beaker of mass 400 g contains 500 g of water at 27 . The beaker is heated reversibly so that the temperature of the beaker and water rise gradually to 57 . Find the change in entropy of the beaker and water together.arrow_forwardTwo moles of a monatomic ideal gas such as oxygen is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with a pressure of 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state. (c) Find work done by the gas in the process. (d) Find the change in internal energy in the process. Assume Cv=5R and Cp=Cv+R for the diatomic ideal gas in the conditions given.arrow_forwardA certain gasoline engine has an efficiency of 30.0%. What would the hot reservoir temperature be for a Carnot engine having that eficiency, if it operates with a cold reservoir temperature of 200°C?arrow_forward
- Discuss the change in entropy of a gas that expands (a) at constant temperature and (b) adiabatically.arrow_forwardHow could you design a Carnot engine with 100% efficiency?arrow_forwardWhat is the entropy change of 10 g of steam at 100 when it condenses to water at the same temperature?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY