
(III) The operation of a diesel engine can be idealized by the cycle shown in Fig. 20-18. Air is drawn into the cylinder during the intake stroke (not part of the idealized cycle). The air is compressed adiabatically, path ab. At point b diesel fuel is injected into the cylinder which immediately burns since the temperature is very high. Combustion is slow, and during the first part of the power stroke, the gas expands at (nearly) constant pressure, path bc. After burning, the rest of the power stroke is adiabatic, path ed. Path da corresponds to the exhaust stroke. (a) Show that, for a quasistatic reversible engine undergoing this cycle using an ideal gas, the ideal efficiency is
where
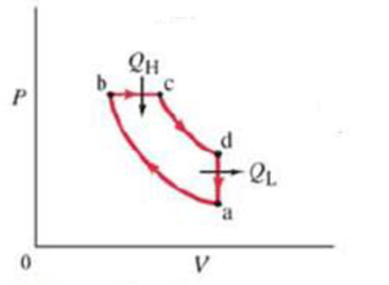
FIGURE 20-18
Problem 7.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Physics for Science and Engineering With Modern Physics, VI - Student Study Guide
Additional Science Textbook Solutions
Microbiology: An Introduction
College Physics: A Strategic Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Essential Biology (7th Edition)
- Does the entropy increase for a Carnot engine for each cycle?arrow_forwardA Carnot engine is used to measure the temperature of a heat reservoir. The engine operates between the heat reservoir and a reservoir consisting of water at its triple point. (a) If 400 J per cycle are removed from the heat reservoir while 200 J per cycle are deposited in the triple-point reservoir, what is the temperature of the heat reservoir? (b) If 400 J per cycle are removed from the triple-point reservoir while 200 J per cycle are deposited in the heat reservoir, what is the temperature of the heat reservoir?arrow_forwardThe energy output of a heat pump is greater than the energy used to operate the pump. Why doesn't this statement violate the first law of thermodynamics?arrow_forward
- For the Carnot cycle of Figure 4.12, what is the entropy change of the hot reservoir, the cold reservoir, and the universe? Figure 4.11 The four processes of the Carnot cycle. The working substance is assumed to be an ideal gas whose thermodynamic path MNOP is represented in Figure 4.12. Figure 4.12 The total work done by the gas in the Carnot cycle is shown and given by the area enclosed by the loop MNOPM.arrow_forwardA Carnot engine operates between 550 and 20 baths and produces 300 kJ of energy in each cycle. Find the change in entropy of the (a) hot bath and (b) cold bath, in each Carnot cycle?arrow_forwardHow could you design a Carnot engine with 100% efficiency?arrow_forward
- In an isochoric process, heat is added to 10 mol of monoatomic ideal gas whose temperature increases from 273 to 373 K. What is the entropy change of the gas?arrow_forwardA certain gasoline engine has an efficiency of 30.0%. What would the hot reservoir temperature be for a Carnot engine having that eficiency, if it operates with a cold reservoir temperature of 200°C?arrow_forwardCheck Your Understanding A Carnot engine operates between reservoirs at 400 and 30 . (a) What is the efficiency of the engine? (b) If the engine does 5.0 J of work per cycle, how much heat per cycle does it absorb from the high-temperature reservoir? (c) How much heat per cycle does it exhaust to the cold-temperature reservoir? (d) What temperatures at the cold reservoir would give the minimum and maximum efficiency?arrow_forward
- A Carnot cycle working between 100 and 30 is used to drive a refrigerator between 10 and 30 . How much energy must the Carnot engine produce per second so that the refrigerator is able to discard 10 J of energy per second?arrow_forwardShow that the coefficients of performance of refrigerators and heat pumps are related by COPref=COPhp1. Start with the definitions of the COP s and the conservation of energy relationship between Qh, QC, and W.arrow_forwardSuppose 20 g of ice at 0 is added to 300 g of water at 60 . What is the total change in entropy of the mixture after it reaches thermal equilibrium?arrow_forward

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning



