
Concept explainers
(a)
Interpretation: Primary, secondary and tertiary hydrogen in below structure along with IUPAC name should be labeled.
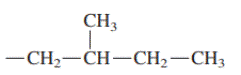
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
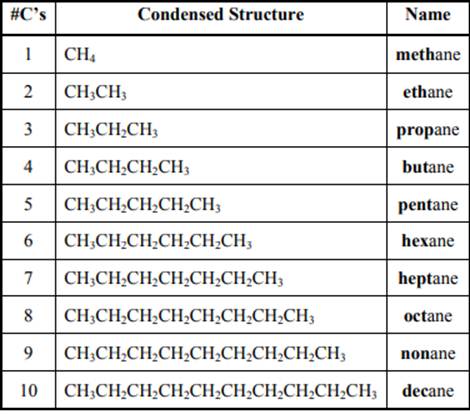
Beside the IUPAC names there are certain common names. The common prefixes include prefixes “iso-” and “neo-”. For example, isobutane is common name used popularly for
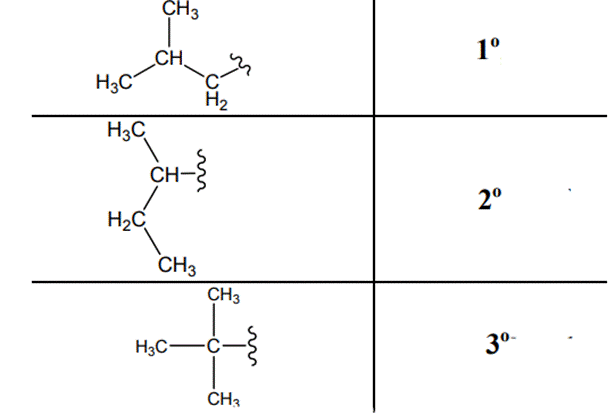
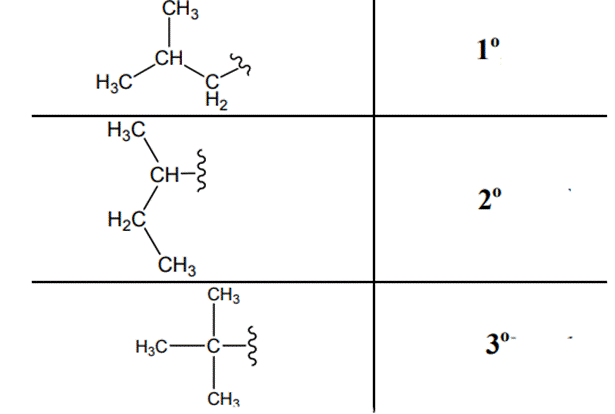
The carbon linked to one alkyl/carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
(b)
Interpretation: Primary, secondary and tertiary hydrogen in below structure along with IUPAC name should be labeled.
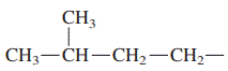
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
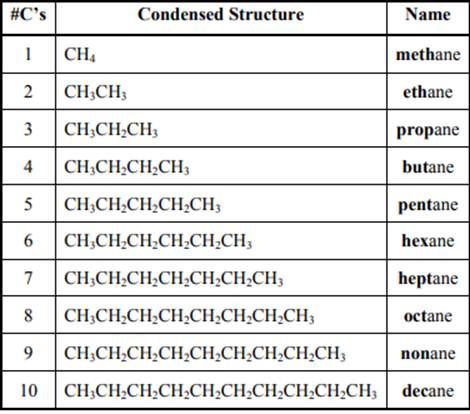
Beside the IUPAC names there are certain common names. The common prefixes include prefixes “iso-” and “neo-”. For example, isobutane is common name used popularly for
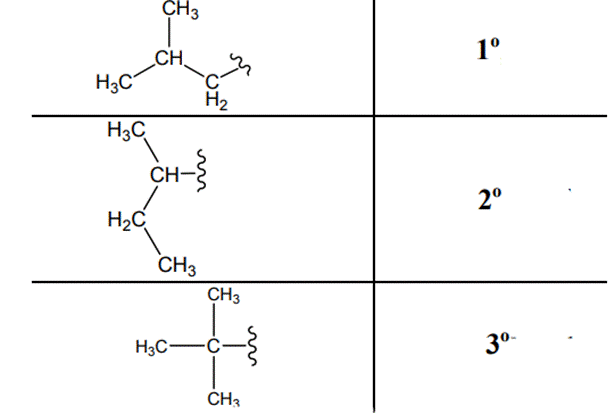
The carbon linked to one alkyl/carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
(c)
Interpretation: Primary, secondary and tertiary hydrogen in below structure along with IUPAC name should be labeled.
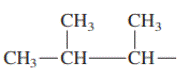
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
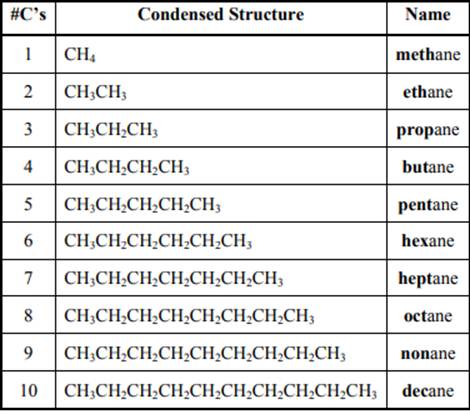
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes” iso-“ and “neo-“ .For example isobutane is common name used popularly for
The carbon linked to one alkyl / carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
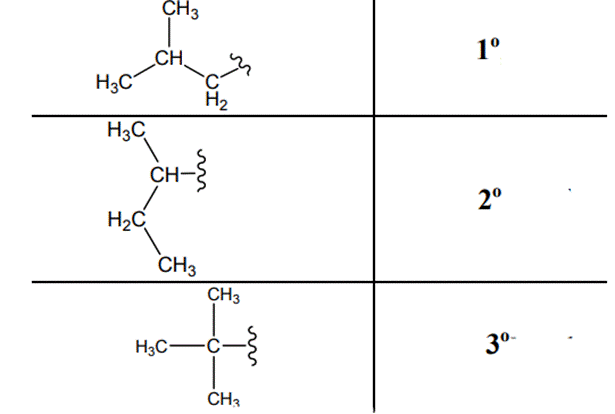
(d)
Interpretation: Primary, secondary and tertiary hydrogen in below structure along with IUPAC name should be labeled.
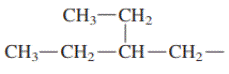
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
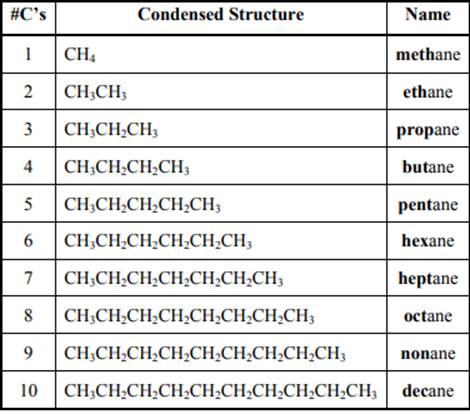
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes” iso-“ and “neo-“ .For example isobutane is common name used popularly for
The carbon linked to one alkyl / carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
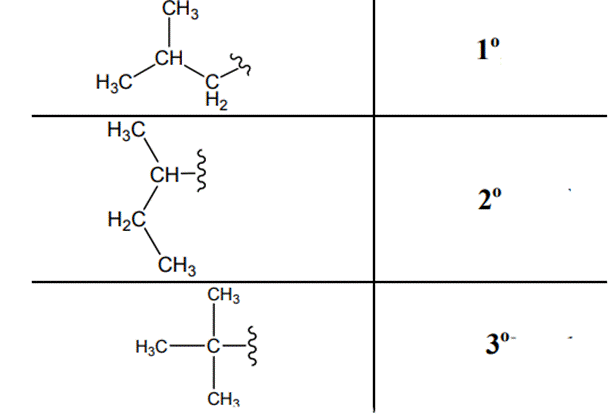
(e)
Interpretation: Primary, secondary and tertiary hydrogen in below structure along with IUPAC name should be labeled.
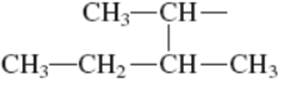
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
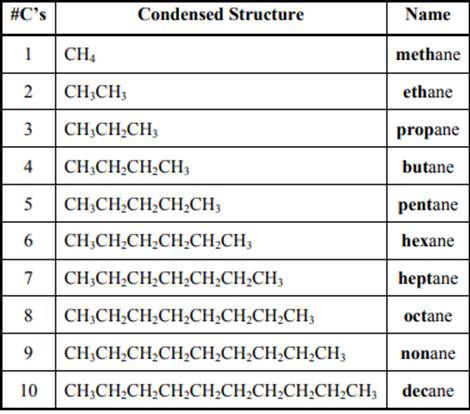
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes” iso-“ and “neo-“ .For example isobutane is common name used popularly for
The carbon linked to one alkyl / carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
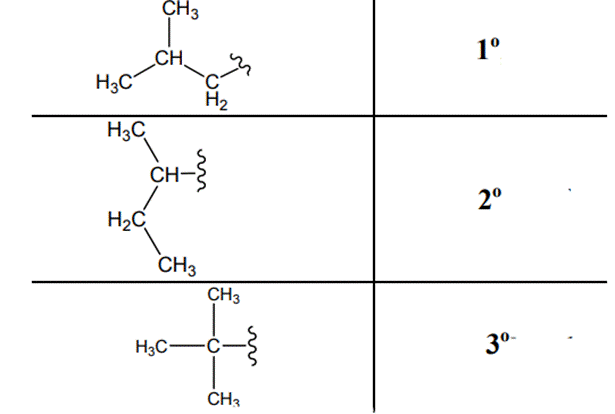
(f)
Interpretation: Primary, secondary and tertiary hydrogen in below structure along with IUPAC name should be labeled.
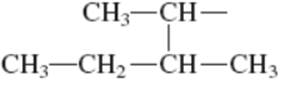
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
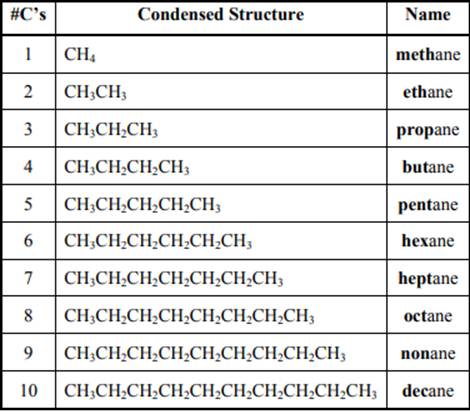
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes” iso-“ and “neo-“ .For example isobutane is common name used popularly for
The carbon linked to one alkyl / carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- What is the mechanism for this?arrow_forward21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forward
- What is the major enolate formed when treated with LDA? And why that one?arrow_forward4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forward
- Indicate the processes in the dismutation of Cu2O.arrow_forward1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forward
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

