
Concept explainers
(a)
Interpretation: Primary, secondary and tertiary hydrogen in structure of ethane should be labeled.
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
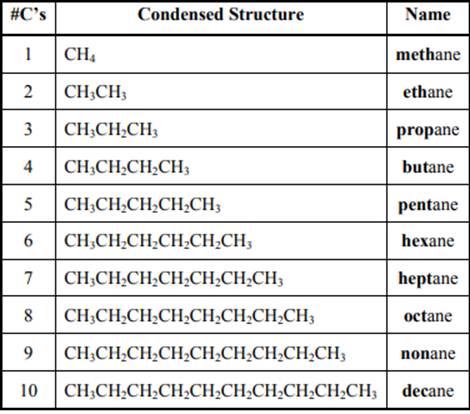
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
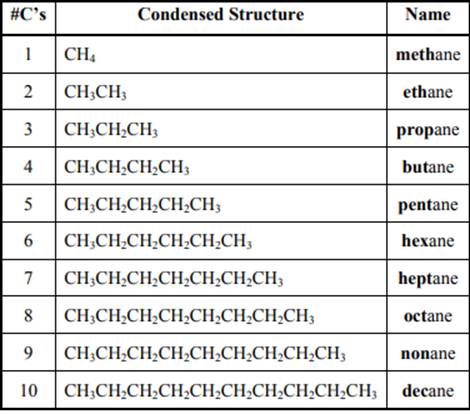
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes “iso-” and “neo-”.For example, isobutane is common name used popularly for
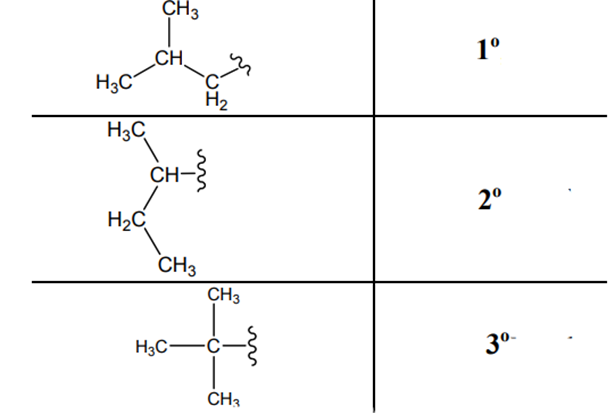
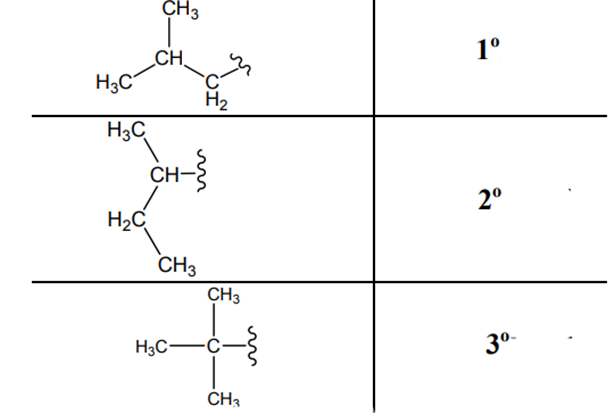
The carbon linked to one alkyl/carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
(b)
Interpretation: Primary, secondary and tertiary hydrogen in structure of pentane should be labeled.
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
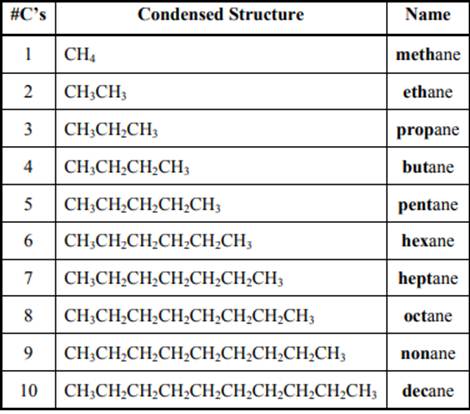
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes “iso-” and “neo-”.For example, isobutane is common name used popularly for
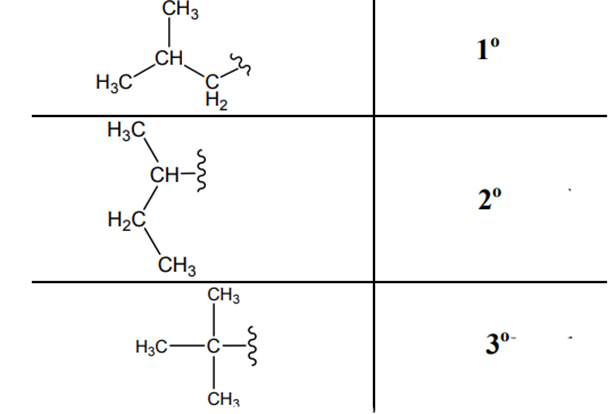
The carbon linked to one alkyl/carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
(c)
Interpretation: Primary, secondary and tertiary hydrogen in structure of
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes “iso-” and “neo-”. For example, isobutane is common name used popularly for
The carbon linked to one alkyl/carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
These are indicated below:
(d)
Interpretation: Primary, secondary and tertiary carbons in structure of
Concept introduction: In accordance with IUPAC convention longest chain can be found from either direction provided it is longest and digits indicate the position of carbon or the position of branched alkyl chain in cases of branched hydrocarbons. All the side chains are named in alphabetical order.
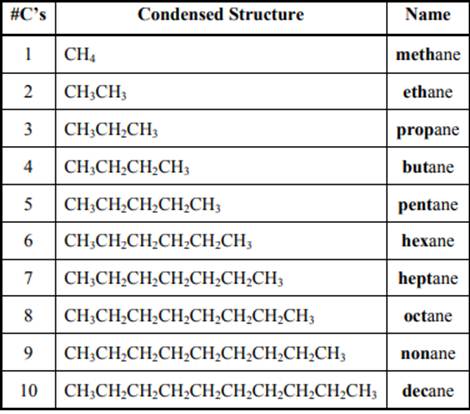
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
Beside the IUPAC names there are certain common names. The common prefixes used include prefixes “iso-” and “neo-”. For example, isobutane is common name used popularly for
The carbon linked to one alkyl/carbon while other two
The carbon linked to two alkyl /carbons and one
The carbon linked to three alkyl groups/carbons and no
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- How does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forward
- Draw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forward
- pls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





