
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 41P
- a. List the following carboxylic acids in order from strongest acid to weakest acid:
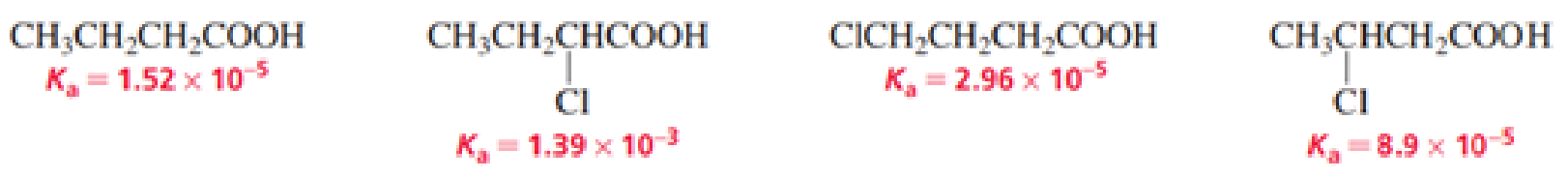
- b. How does the presence of an electronegative substituent such as Cl affect the acidity of a
carboxylic acid ? - c. How does the location of the substituent affect the acidity of the carboxylic acid?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Predict the major product(s) for each of the following reactions.
HBr (1 equiv.)
peroxide, A
a.
b.
NBS,
peroxide, A
In addition to the separation techniques used in this lab (magnetism, evaporation, and filtering), there are other commonly used separation techniques. Some of these techniques are:Distillation – this process is used to separate components that have significantly different boiling points. The solution is heated and the lower boiling point substance is vaporized first. The vapor can be collected and condensed and the component recovered as a pure liquid. If the temperature of the mixture is then raised, the next higher boiling component will come off and be collected. Eventually only non-volatile components will be left in the original solution.Centrifugation – a centrifuge will separate mixtures based on their mass. The mixture is placed in a centrifuge tube which is then spun at a high speed. Heavier components will settle at the bottom of the tube while lighter components will be at the top. This is the technique used to separate red blood cells from blood plasma.Sieving – this is…
Briefly describe a eutectic system.
Chapter 2 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 2.1 - Which of the following are not acids? CH3COOH CO2...Ch. 2.1 - Draw the products of the acidbase reaction when a....Ch. 2.1 - a.What is the conjugate acid of each of the...Ch. 2.2 - a. Which is a stronger acid, one with a pKa of 5.2...Ch. 2.2 - Prob. 5PCh. 2.2 - Antacids are compounds that neutralize stomach...Ch. 2.2 - Are the following body fluids acidic or basic? a....Ch. 2.3 - Draw the conjugate acid of each of the following:...Ch. 2.3 - a. Write an equation showing CH3OH reacting as an...Ch. 2.3 - Prob. 10P
Ch. 2.3 - a. Which is a stronger base, CH3COO or HCOO? (The...Ch. 2.3 - Using the pKa values in Section 2.3, rank the...Ch. 2.4 - Prob. 13PCh. 2.5 - Prob. 14PCh. 2.5 - Ethyne has a pKa value of 25, water has a pKa...Ch. 2.5 - Which of the following bases can remove a proton...Ch. 2.6 - List the ions (CH3, NH2, HO, and F) in order from...Ch. 2.6 - List the carbanions shown in the margin in order...Ch. 2.6 - Which is a stronger acid?Ch. 2.6 - a. Draw the products of the following reactions: A...Ch. 2.6 - List the halide ions (F, Cl, Br, and I) in order...Ch. 2.6 - a. Which is more electronegative, oxygen or...Ch. 2.6 - Which is a stronger acid? a. HCl or HBr b....Ch. 2.6 - a. Which of the halide ions (F, Cl, Br, and I) is...Ch. 2.6 - Which is a stronger base? a. H2O or HO b. H2O or...Ch. 2.7 - Which is a stronger acid? a. CH3OCH2CH2OH or...Ch. 2.7 - Which is a stronger base?Ch. 2.8 - Fosamax has six acidic groups. The structure of...Ch. 2.8 - Which is a stronger acid? Why?Ch. 2.10 - For each of the following compounds (shown in...Ch. 2.10 - Prob. 33PCh. 2.11 - Write the equation that shows how a buffer made by...Ch. 2.11 - What products are formed when each of the...Ch. 2 - a. List the following alcohols in order from...Ch. 2 - Which is a stronger base? a. HS or HO b. CH3O or...Ch. 2 - Prob. 40PCh. 2 - a. List the following carboxylic acids in order...Ch. 2 - For the following compound, a. draw its conjugate...Ch. 2 - List the following compounds in order from...Ch. 2 - For each of the following compounds, draw the form...Ch. 2 - Give the products of the following acidbase...Ch. 2 - Prob. 46PCh. 2 - For each compound, indicate the atom that is most...Ch. 2 - Tenormin, a member of the group of drugs known as...Ch. 2 - From which acids can HO remove a proton in a...Ch. 2 - Prob. 50PCh. 2 - Which is a stronger acid? a. CH29CHCOOH or...Ch. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - How could you separate a mixture of the following...Ch. 2 - Prob. 1PCh. 2 - Prob. 2PCh. 2 - Draw the products of the following acidbase...Ch. 2 - Prob. 4PCh. 2 - Prob. 5PCh. 2 - Prob. 6PCh. 2 - Prob. 7PCh. 2 - Prob. 8PCh. 2 - Prob. 9PCh. 2 - Prob. 10PCh. 2 - Prob. 11PCh. 2 - Prob. 12PCh. 2 - Prob. 13PCh. 2 - Prob. 14P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- man Campus Depa (a) Draw the three products (constitutional isomers) obtained when 2-methyl-3-hexene reacts with water and a trace of H2SO4. Hint: one product forms as the result of a 1,2-hydride shift. (1.5 pts) This is the acid-catalyzed alkene hydration reaction.arrow_forwardNonearrow_forward. • • Use retrosynthesis to design a synthesis Br OHarrow_forward
- 12. Choose the best diene and dienophile pair that would react the fastest. CN CN CO₂Et -CO₂Et .CO₂Et H3CO CO₂Et A B C D E Farrow_forward(6 pts - 2 pts each part) Although we focused our discussion on hydrogen light emission, all elements have distinctive emission spectra. Sodium (Na) is famous for its spectrum being dominated by two yellow emission lines at 589.0 and 589.6 nm, respectively. These lines result from electrons relaxing to the 3s subshell. a. What is the photon energy (in J) for one of these emission lines? Show your work. b. To what electronic transition in hydrogen is this photon energy closest to? Justify your answer-you shouldn't need to do numerical calculations. c. Consider the 3s subshell energy for Na - use 0 eV as the reference point for n=∞. What is the energy of the subshell that the electron relaxes from? Choose the same emission line that you did for part (a) and show your work.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY