
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.96P
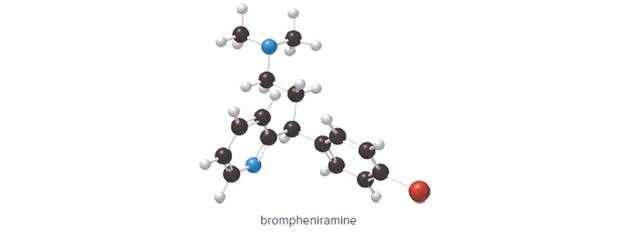
(a) What is the chemical formula for brompheniramine, an antihistamine used to relieve the runny nose and sneezing associated with allergies? (b) Which element in brompheniramine has valence electrons that are farthest from the nucleus?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6
Which of the following are likely to be significant resonance structures of a resonance hybrid? Draw another resonance
structure for each of the compounds you select as being a resonance form. (A
Br:
Br:
A
B
C
D
E
Write the systematic (IUPAC) name for the following organic molecules.
Note for advanced students: you do not need to include any E or Z prefixes in your names.
Br
structure
Br
Br
Oweu
Conservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.
Chapter 2 Solutions
General, Organic, & Biological Chemistry
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used ineach example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.7PCh. 2.2 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - For the given atom: (a) determine the number of...
Ch. 2.2 - How many protons, neutrons, and electrons are...Ch. 2.2 - What is the mass number of an atom that contains...Ch. 2.3 - For each atom give the following information: [1]...Ch. 2.3 - Magnesium has three isotopes that contain 12, 13,...Ch. 2.3 - Prob. 2.15PCh. 2.3 - Calculate the atomic weight of each element given...Ch. 2.4 - Prob. 2.17PCh. 2.4 - Prob. 2.18PCh. 2.4 - Identify the element fitting each description. an...Ch. 2.4 - Identify each highlighted element in the periodic...Ch. 2.5 - How many electrons are present in each shell,...Ch. 2.6 - What element has each electronic configuration? a....Ch. 2.6 - What element(s) in the first and second period fit...Ch. 2.6 - Draw an orbital diagram for each element; (a)...Ch. 2.6 - Give the electronic configuration for each element...Ch. 2.7 - Prob. 2.26PCh. 2.7 - Determine the number of valence electrons and give...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Give the electron-dot symbol for each element: (a)...Ch. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Prob. 2.31PCh. 2.8 - (a) Which of the indicated atoms has the smaller...Ch. 2 - Identify the elements used in each example of...Ch. 2 - Write a chemical formula for each example of...Ch. 2 - Give the name of the elements in each group of...Ch. 2 - What element(s) are designated by each symbol or...Ch. 2 - Does each chemical formula represent an element or...Ch. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.39PCh. 2 - Prob. 2.40PCh. 2 - Give all of the terms that apply to each...Ch. 2 - Give all of the terms that apply to each...Ch. 2 - Give the following information about the atom...Ch. 2 - Give the following information about the atom...Ch. 2 - Prob. 2.45PCh. 2 - Prob. 2.46PCh. 2 - Label each region on the periodic table. Noble...Ch. 2 - Identify each highlighted element in the periodic...Ch. 2 - Prob. 2.49PCh. 2 - Prob. 2.50PCh. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - Prob. 2.53PCh. 2 - Prob. 2.54PCh. 2 - Prob. 2.55PCh. 2 - Prob. 2.56PCh. 2 - How many protons, neutrons, and electrons are...Ch. 2 - Give the number of protons, neutrons, and...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Calculate the atomic weight of silver, which has...Ch. 2 - Calculate the atomic weight of antimony, which has...Ch. 2 - Prob. 2.63PCh. 2 - What is the maximum number of electrons that can...Ch. 2 - Prob. 2.65PCh. 2 - Use an orbital diagram to write the electronic...Ch. 2 - Prob. 2.67PCh. 2 - For each element in Problem 2.66: Write out the...Ch. 2 - Prob. 2.69PCh. 2 - Prob. 2.70PCh. 2 - Give the total number of electrons, the number of...Ch. 2 - Give the total number of electrons, the number of...Ch. 2 - Prob. 2.73PCh. 2 - Prob. 2.74PCh. 2 - Prob. 2.75PCh. 2 - Prob. 2.76PCh. 2 - Prob. 2.77PCh. 2 - Prob. 2.78PCh. 2 - Prob. 2.79PCh. 2 - Prob. 2.80PCh. 2 - Prob. 2.81PCh. 2 - Prob. 2.82PCh. 2 - Prob. 2.83PCh. 2 - Prob. 2.84PCh. 2 - Prob. 2.85PCh. 2 - Prob. 2.86PCh. 2 - Prob. 2.87PCh. 2 - Prob. 2.88PCh. 2 - Rank the atoms in each group in order of...Ch. 2 - Prob. 2.90PCh. 2 - Prob. 2.91PCh. 2 - Prob. 2.92PCh. 2 - Prob. 2.93PCh. 2 - Prob. 2.94PCh. 2 - Prob. 2.95PCh. 2 - (a) What is the chemical formula for...Ch. 2 - Answer the following questions about the...Ch. 2 - Platinum is a precious metal used in a wide...Ch. 2 - Prob. 2.99PCh. 2 - Answer the following questions about the...Ch. 2 - Prob. 2.101CPCh. 2 - Prob. 2.102CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What impact would adding twice as much Na2CO3 than required for stoichiometric quantities have on the quantity of product produced? Initial results attachedarrow_forwardGiven that a theoretical yield for isolating Calcium Carbonate in this experiment would be 100%. From that information and based on the results you obtained in this experiment, describe your success in the recovery of calcium carbonate and suggest two possible sources of error that would have caused you to not obtain 100% yield. Results are attached form experimentarrow_forward5) Calculate the flux of oxygen between the ocean and the atmosphere(2 pts), given that: (from Box 5.1, pg. 88 of your text): Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturated What is flux if the temperature is 10°C ? (2 pts) (Hint: use the same density in your calculations). Why do your calculated values make sense (or not) based on what you know about the relationship between gas solubility and temperature (1 pt)?arrow_forward
- The following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward(ME EX2) Prblms Can you please explain problems to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
NEET Chemistry | Group 14 Carbon Family | Theory & Problem Solving | In English | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=enOGIrcHh54;License: Standard YouTube License, CC-BY