
Interpretation:
The given table is to be completed for the given neutral elements.
Concept introduction:
The electrons in the atoms revolve around the nucleus in different orbits. The nucleus of the atom has two particles, protons and neutrons. The number of proton is the
Answer to Problem 2.45P
The complete table is as shown below.
| Element Symbol | Atomic number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons | |
| a | ||||||
| b | ||||||
| c | ||||||
| d | ||||||
| e | ||||||
| f | ||||||
| g | ||||||
| h |
Explanation of Solution
All the elements are assigned a letter symbol from their name. The symbol is the first or first two letters from the name of the element.
The atomic number of an element is the number of protons in its atom.
The mass number of the element is the sum of the numbers of protons and neutrons present in the nucleus of the atom.
The protons and neutrons are present in the nucleus of the atom and electrons revolve around the nucleus. The number of electrons and protons are equal in a neutral atom as the electrons and protons are charged bodies.
To complete the given table, the points given above are to be considered.
For the case (a), the data given is the
So,
The symbol
The number of neutrons is calculated by the formula given below.
Substitute the given values in the above formula.
Hence, carbon with element symbol
For the case (b) the data given is its mass number
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
The number of neutrons can be calculated by the formula given below.
Substitute the given values in the formula below.
So, the element is phosphorus as its atomic number is
For the case (c), the data given is its number of neutrons
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
Its number of neutrons is
Substitute the given values in the above formula
So, the element is zinc as its atomic number is
For the case (d), the data given is the symbol of element
So, the symbol
The number of neutrons can be calculated by the formula given below.
Substitute the given values in the above formula.
So, the magnesium element with symbol
For the case (e), the data given is its number of neutrons
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
Its atomic number is
Substitute the given values in the above formula
So the element is iodine as its atomic number is
For the case (f), the data given is its atomic number
So, the atomic number is equal to the number of protons and electrons in the atom. Its atomic number is
The mass number of the element can be calculated by the formula given below.
Substitute the given values in the formula below.
So the element is beryllium as its atomic number is
protons and 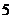 neutrons. Its atomic number is
neutrons. Its atomic number is
and mass number is 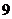
For the case (g) the data given is its atomic number
So, the atomic number is equal to the number of protons and electrons in the atom. Its atomic number is
The number of neutron of the element can be calculated by the formula given below.
Substitute the given values in the above formula
So the element is zirconium as its atomic number is
For the case (h), the number of neutrons is
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
The mass number can be calculated by the formula given below.
Substitute the given values in the formula below.
So the element is sulfur as its atomic number is
The table is completed with the symbols of elements and the information about their atoms and nucleus.
Want to see more full solutions like this?
Chapter 2 Solutions
General, Organic, & Biological Chemistry
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





