
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Chapter 2.3, Problem 2.15P
Interpretation Introduction
Interpretation:
The species with the highest mass number from the following isotope should be determined:
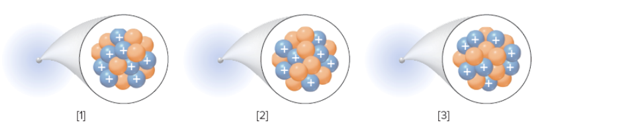
Concept Introduction:
Isotopes of an element have same
Interpretation Introduction
Interpretation:
The number of electrons in the neutral atom of each element should be determined:
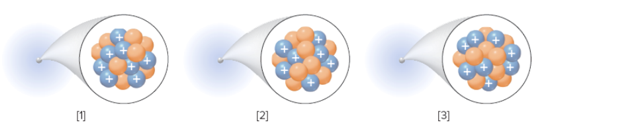
Concept Introduction:
An
Interpretation Introduction
Interpretation:
The isotopic symbol for each element should be determined:
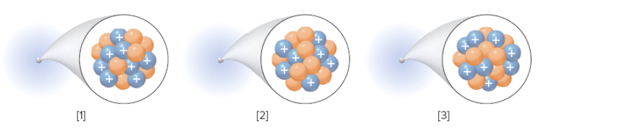
Concept Introduction:
An isotope of an element have same atomic number but different mass number.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the Hofmann product of the dehydroiodination of this alkyl iodide.
☐ :
+
Explanation
Check
esc
F1
2
3
I
88
%
5
F5
I.
X
©
tBuOK
Click and drag to sta
drawing a structure.
© 2025 McGraw Hill LLC. All Rights Reserved. Te
BI
BB
F6
W
E
R
Y
S
H
K
Can I please get help with this graph, if you could show exactly where it needs to pass through please.
Draw the condensed structure of 1,3-dihydroxy-2-pentanone.
Explanation
Check
Click anywhere to draw the first
atom of your structure.
Х
C
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of use
+
Chapter 2 Solutions
General, Organic, & Biological Chemistry
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used ineach example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.7PCh. 2.2 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - For the given atom: (a) determine the number of...
Ch. 2.2 - How many protons, neutrons, and electrons are...Ch. 2.2 - What is the mass number of an atom that contains...Ch. 2.3 - For each atom give the following information: [1]...Ch. 2.3 - Magnesium has three isotopes that contain 12, 13,...Ch. 2.3 - Prob. 2.15PCh. 2.3 - Calculate the atomic weight of each element given...Ch. 2.4 - Prob. 2.17PCh. 2.4 - Prob. 2.18PCh. 2.4 - Identify the element fitting each description. an...Ch. 2.4 - Identify each highlighted element in the periodic...Ch. 2.5 - How many electrons are present in each shell,...Ch. 2.6 - What element has each electronic configuration? a....Ch. 2.6 - What element(s) in the first and second period fit...Ch. 2.6 - Draw an orbital diagram for each element; (a)...Ch. 2.6 - Give the electronic configuration for each element...Ch. 2.7 - Prob. 2.26PCh. 2.7 - Determine the number of valence electrons and give...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Give the electron-dot symbol for each element: (a)...Ch. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Prob. 2.31PCh. 2.8 - (a) Which of the indicated atoms has the smaller...Ch. 2 - Identify the elements used in each example of...Ch. 2 - Write a chemical formula for each example of...Ch. 2 - Give the name of the elements in each group of...Ch. 2 - What element(s) are designated by each symbol or...Ch. 2 - Does each chemical formula represent an element or...Ch. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.39PCh. 2 - Prob. 2.40PCh. 2 - Give all of the terms that apply to each...Ch. 2 - Give all of the terms that apply to each...Ch. 2 - Give the following information about the atom...Ch. 2 - Give the following information about the atom...Ch. 2 - Prob. 2.45PCh. 2 - Prob. 2.46PCh. 2 - Label each region on the periodic table. Noble...Ch. 2 - Identify each highlighted element in the periodic...Ch. 2 - Prob. 2.49PCh. 2 - Prob. 2.50PCh. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - Prob. 2.53PCh. 2 - Prob. 2.54PCh. 2 - Prob. 2.55PCh. 2 - Prob. 2.56PCh. 2 - How many protons, neutrons, and electrons are...Ch. 2 - Give the number of protons, neutrons, and...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Calculate the atomic weight of silver, which has...Ch. 2 - Calculate the atomic weight of antimony, which has...Ch. 2 - Prob. 2.63PCh. 2 - What is the maximum number of electrons that can...Ch. 2 - Prob. 2.65PCh. 2 - Use an orbital diagram to write the electronic...Ch. 2 - Prob. 2.67PCh. 2 - For each element in Problem 2.66: Write out the...Ch. 2 - Prob. 2.69PCh. 2 - Prob. 2.70PCh. 2 - Give the total number of electrons, the number of...Ch. 2 - Give the total number of electrons, the number of...Ch. 2 - Prob. 2.73PCh. 2 - Prob. 2.74PCh. 2 - Prob. 2.75PCh. 2 - Prob. 2.76PCh. 2 - Prob. 2.77PCh. 2 - Prob. 2.78PCh. 2 - Prob. 2.79PCh. 2 - Prob. 2.80PCh. 2 - Prob. 2.81PCh. 2 - Prob. 2.82PCh. 2 - Prob. 2.83PCh. 2 - Prob. 2.84PCh. 2 - Prob. 2.85PCh. 2 - Prob. 2.86PCh. 2 - Prob. 2.87PCh. 2 - Prob. 2.88PCh. 2 - Rank the atoms in each group in order of...Ch. 2 - Prob. 2.90PCh. 2 - Prob. 2.91PCh. 2 - Prob. 2.92PCh. 2 - Prob. 2.93PCh. 2 - Prob. 2.94PCh. 2 - Prob. 2.95PCh. 2 - (a) What is the chemical formula for...Ch. 2 - Answer the following questions about the...Ch. 2 - Platinum is a precious metal used in a wide...Ch. 2 - Prob. 2.99PCh. 2 - Answer the following questions about the...Ch. 2 - Prob. 2.101CPCh. 2 - Prob. 2.102CP
Knowledge Booster
Similar questions
- 0.500 moles of NOCl are placed into a 1.00 L vessesl at 700K and after the system comes to equilibrium, the consentration of NOCl is 0.440 M. Calculate the equilibrium constant Kc for the reaction: 2NOCL (g) --> 2NO (g) + Cl2 (g)arrow_forwardWhat is the hydronium ion concentration in a solution of water that has a hydroxide ion concentrationof 1.0 x 10-2 M?arrow_forwardIdentify conjugate acid-base pairs in the following reactions:HBr (aq) + H2O (l) ⇌ H3O+ (aq) + Br- (aq) - OH (aq) + CH3COOH (aq) ⇌ H2O (l) + CH3COO- (aq)arrow_forward
- 4:45 PM Tue Apr 1 K 77% Problem 9 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. :0: H Select to Add Arrows HI CH3OH H+ ·HO CH3OH, H+ 0:0 H H Select to Add Arrows tion Versirate CH3OH, H* Select to Draw Productarrow_forwardCan I please get help with this graph? If you can show exactly where it needs to pass through.arrow_forwardG 1. PPh3, THF 2. 3. LiH, THF ' THF H Harrow_forward
- Please EnCircle or Fill-In your Choice CLEARLY: 21. Please Sketch the intermediates for each step below. Draw the Product which would result from the following series of reactions. Name each Type of Rx: 1. Br2, FeBr3 2. Mg, ether 3. ethylene oxide 4. H₂O+ 5. PBr3 6. Mg, ether 7. 8. H3O+, heat (-H₂O 9. HF ?arrow_forwardCan I please get help with this question. All required information should be in data table.arrow_forwardesc For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. Major Product: Explanation Check C ☐ + X NaOH Br F1 F2 80 F3 F4 F5 F6 1 ! @ 2 3 $ 4 % 5 Q W LU E S D A F7 * C Click and dr drawing a 2025 McGraw Hill LLC. All Rights Reserv ►II F8 4 F9 6 7 8 9 R T Y U LL F G H Jarrow_forward
- Calculate equilibrium concentrations for the following reaction:N2 (g) + O2 (g) ⇋ 2 NO (g) Kc = 0.10 at 2273K initially [N2] = 0.200M; [O2] = 0.200arrow_forwardFor each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardThe following reaction is run in which the initial conditions include only methane (CH4) at a concentration of0.115 M. Once equilibrium was established, the concentration of acetylene (C2H2) was measured to be 0.035M. What is the value of the equilibrium constant, K?2 CH4 (g) ⇋ C2H2 (g) + 3 H2 (g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning