
Interpretation:
The given table is to be completed for the given neutral elements.
Concept introduction:
The electrons in the atoms revolve around the nucleus in different orbits. The nucleus of the atom has two particles, protons and neutrons. The number of proton is the
Answer to Problem 2.45P
The complete table is as shown below.
| Element Symbol | Atomic number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons | |
| a | ||||||
| b | ||||||
| c | ||||||
| d | ||||||
| e | ||||||
| f | ||||||
| g | ||||||
| h |
Explanation of Solution
All the elements are assigned a letter symbol from their name. The symbol is the first or first two letters from the name of the element.
The atomic number of an element is the number of protons in its atom.
The mass number of the element is the sum of the numbers of protons and neutrons present in the nucleus of the atom.
The protons and neutrons are present in the nucleus of the atom and electrons revolve around the nucleus. The number of electrons and protons are equal in a neutral atom as the electrons and protons are charged bodies.
To complete the given table, the points given above are to be considered.
For the case (a), the data given is the
So,
The symbol
The number of neutrons is calculated by the formula given below.
Substitute the given values in the above formula.
Hence, carbon with element symbol
For the case (b) the data given is its mass number
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
The number of neutrons can be calculated by the formula given below.
Substitute the given values in the formula below.
So, the element is phosphorus as its atomic number is
For the case (c), the data given is its number of neutrons
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
Its number of neutrons is
Substitute the given values in the above formula
So, the element is zinc as its atomic number is
For the case (d), the data given is the symbol of element
So, the symbol
The number of neutrons can be calculated by the formula given below.
Substitute the given values in the above formula.
So, the magnesium element with symbol
For the case (e), the data given is its number of neutrons
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
Its atomic number is
Substitute the given values in the above formula
So the element is iodine as its atomic number is
For the case (f), the data given is its atomic number
So, the atomic number is equal to the number of protons and electrons in the atom. Its atomic number is
The mass number of the element can be calculated by the formula given below.
Substitute the given values in the formula below.
So the element is beryllium as its atomic number is
protons and 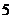 neutrons. Its atomic number is
neutrons. Its atomic number is
and mass number is 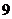
For the case (g) the data given is its atomic number
So, the atomic number is equal to the number of protons and electrons in the atom. Its atomic number is
The number of neutron of the element can be calculated by the formula given below.
Substitute the given values in the above formula
So the element is zirconium as its atomic number is
For the case (h), the number of neutrons is
So, the atomic number is equal to the number of protons and electrons in the atom. This atom has
The mass number can be calculated by the formula given below.
Substitute the given values in the formula below.
So the element is sulfur as its atomic number is
The table is completed with the symbols of elements and the information about their atoms and nucleus.
Want to see more full solutions like this?
Chapter 2 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Indicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardSynthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forward
- Indicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardIdentify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forwardWhat is single-point calibration? Provide an example.arrow_forward
- Draw the major product formed via an E1 pathway.arrow_forwardPart 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forwardConsider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forward
- julietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardThe electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forwardHello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





