
Concept explainers
(a)
To identify: The potential hydrogen bond donors and accepters present in Fig.1.
Concept introduction: Hydrogen bonds are formed between polar molecules. It is an intermolecular attraction that forms between partially positive hydrogen atoms of a polar molecule with a partially negative atom of another polar molecule. In general chemical formulation, a hydrogen bond is explained as D–H···A, where D–H is a hydrogen bond “donor group” and A is considered as a hydrogen bond accepter group or an atom.
(a)
Answer to Problem 1E
Correct answer: The potential hydrogen bond donor groups are HN1, H2N at the C2 position, and HN9. The potential hydrogen bond accepter groups are O at the C6 position, N3, and N7.
Explanation of Solution
Pictorial presentation: Fig. 1 shows structure of guanine, where the potential hydrogen bond donors and accepters are identified.
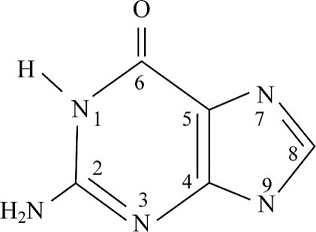
Fig.1: Guanine
The given formula of Fig.1 is identified as Guanine. Here, at the HN1 position, the hydrogen bond is donated and nitrogen acts as a hydrogen bond donor when it is paired with a hydrogen atom. When nitrogen is not paired with a hydrogen atom, it acts as a hydrogen accepter. Therefore, at HN1, H2N at the C2 position, and HN9 portion, the hydrogen bond acts like a donor; O at the C6 position, N3, and N7 portion, the hydrogen bond acts like an acceptor.
(b)
To identify: The potential hydrogen bond donors and acceptors present in Fig.2.
Concept introduction: Hydrogen bonds are formed between polar molecules. It is an intermolecular attraction that forms between partially positive hydrogen atoms of a polar molecule with a partially negative atom of another polar molecule. In general chemical formulation, a hydrogen bond is explained as D–H···A, where D–H is a hydrogen bond “donor group” and A is considered as a hydrogen bond accepter group or atom.
(b)
Answer to Problem 1E
Correct answer: The potential hydrogen bond donor groups are HN1 and H2N at the C4 position. The potential hydrogen bond accepter groups are O at the C2 position, N3.
Explanation of Solution
Pictorial presentation: Fig.2 shows structure of cytosine, where the potential hydrogen bond donors and accepters are identified.
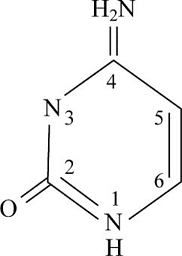
Fig.1: Cytosine
The given formula of Fig.2 is identified as cytosine. Here, at HN1 position, the hydrogen bond is donated as nitrogen acts as the hydrogen bond donor when it is paired with a hydrogen atom. When nitrogen is not paired with a hydrogen atom, it acts as a hydrogen acceptor. Therefore, at HN1 and H2N at the C4 position, the hydrogen bond acts like a donor and O at the C2 position and N3 portion, the hydrogen bond acts like an acceptor.
(c)
To identify: The potential hydrogen bond donors and accepters present in Fig.3.
Concept introduction: Hydrogen bonds are formed between the polar molecules. It is an intermolecular attraction that forms between partially positive hydrogen atoms of a polar molecule with a partially negative atom of another polar molecule. In general chemical formulation, a hydrogen bond is explained as D–H···A, where D–H is a hydrogen bond “donor group” and A is considered as a hydrogen bond accepter group or atom.
(c)
Answer to Problem 1E
Correct answer: The potential hydrogen bond donor groups are H3N+ group and OH group. The potential hydrogen bond accepter groups are COO and OH.
Explanation of Solution
Pictorial presentation: Fig.3 shows structure of serine, where the potential hydrogen bond donors and acceptors are identified.
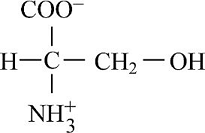
Fig.3: Serine
The given formula of Fig.3 is identified as serine. Here, at H3N+ position, the hydrogen bond is donated as nitrogen acts as a hydrogen bond donor when it is paired with a hydrogen atom. When nitrogen is not paired with a hydrogen atom, it acts as a hydrogen acceptor. Therefore, at H3N+ group and OH group, the hydrogen bond acts like a donor and COO and OH position, the hydrogen bond acts like an acceptor.
Want to see more full solutions like this?
Chapter 2 Solutions
FUNDAMENTALS OF BIOCHEMISTRY WPNG 1-SEME
- Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward+NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
- Which features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forwardLooking at the figure 30-5 what intermolecular forces are present between the substrate and the enzyme and the substrate and cofactors.arrow_forwardprovide short answers to the followings Urgent!arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvatedehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanismfor this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency inany one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and ischaracterized by cardiac and neurological symptoms. One key diagnostic forthis disease is an increased level of pyruvate and α-ketoglutarate in thebloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardDraw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle - (Draw the Mechanism). How many rounds of Krebs will be required to waste all Carbons of Glutamic Acidas CO2?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





