
a)
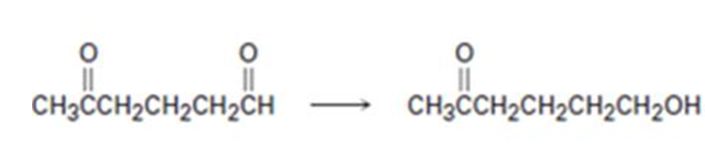
Interpretation:
How to carry out the selective transformation of a ketoaldehyde to ketoalcohol is to be shown.
Concept introduction:
Both
To show:
How to carry out the selective transformation of a ketoaldehyde to ketoalcohol.
b)
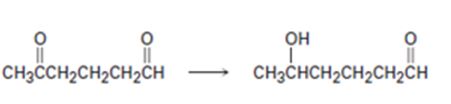
Interpretation:
How to carry out the selective transformation of a diketone to an aldol is to be shown.
Concept introduction:
Both aldehyde and keto groups are reduced by LiAlH4. In order to selectively reduce one keto group, the other keto group is first protected by converting it into an acetal. The protected
To show:
How to carry out the selective transformation of a diketone to an aldol.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry - With Access (Custom)
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
- 1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

