
a)

Interpretation:
How mass spectrometry is useful in distinguishing between 3-methyl-2-hexanone and 4-methyl-2-hexanone is to be stated.
Concept introduction:
In the mass spectrum of organic compounds, normally peaks due to Mc Lafferty rearrangement and α cleavage are generally visible. In Mc Lafferty rearrangement,
To state:
How to distinguish between 3-methyl-2-hexanone and 4-methyl-2-hexanone using mass spectrometry.
b)

Interpretation:
How mass spectrometry is useful in distinguishing between 3-heptanone and 4-heptanone is to be stated.
Concept introduction:
In the mass spectrum of organic compounds, normally peaks due to Mc Lafferty rearrangement and α cleavage are generally visible. In Mc Lafferty rearrangement, aldehydes and ketones that have hydrogens on the γ carbons transfer the hydrogen on the carbon to the carbonyl oxygen and the bond between the α and β carbons is broken to yield an alkene. The charge remains with the oxygen containing fragment. In the α cleavage, the bond between the carbonyl carbon and the α carbon breaks to yield a neutral radical and a resaonance stabilized acyl cation.
To state:
How to distinguish between 3-heptanone and 4-heptanone using mass spectrometry.
c)
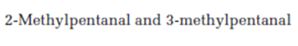
Interpretation:
How mass spectrometry is useful in distinguishing between 2-methylpentanal and 3-methylpentanal is to be stated.
Concept introduction:
In the mass spectrum of organic compounds, normally peaks due to Mc Lafferty rearrangement and α cleavage are generally visible. In Mc Lafferty rearrangement, aldehydes and ketones that have hydrogens on the γ carbons transfer the hydrogen on the carbon to the carbonyl oxygen and the bond between the α and β carbons is broken to yield an alkene. The charge remains with the oxygen containing fragment. In the α cleavage, the bond between the carbonyl carbon and the α carbon breaks to yield a neutral radical and a resaonance stabilized acyl cation.
To show:
How to distinguish between 2-methylpentanal and 3-methylpentanal using mass spectrometry.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry - With Access (Custom)
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





