
Concept explainers
(a)
Interpretation: The structure of the given compound para-toluidine has to be drawn.
Concept introduction:
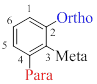
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)
Aryl
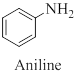
Toluidine is an aryl amine whose structure is similar to aniline except that a methyl group is substituted onto the benzene ring.
(b)
Interpretation: The structure of the given compound meta-cresol has to be drawn.
Concept Introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Phenol is an
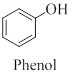
Cresol is a derivative of phenol which contains a methyl group other than the
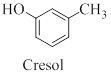
(c)
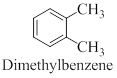
Interpretation: The structure of the given compound para-xylene has to be drawn.
Concept Introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Dimethyl benzene compounds are known as xylene. Depends on the position of methyl group it can be exist as ortho, para and Meta compound.
(d)
Interpretation:
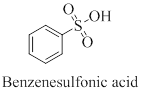
The structure of the given compound ortho-chlorobenzenesulfonic acid has to be drawn.
Concept introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Benzene sulfonic acid is an organosulfur compound with the following structure.
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
- Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forwardAt 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forward
- Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardDraw the skeletal structure of the alkane 4-ethyl-2, 2, 5, 5- tetramethylnonane. How many primary, secondary, tertiary, and quantenary carbons does it have?arrow_forward
- Electronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





