
EBK ORGANIC CHEMISTRY
7th Edition
ISBN: 9780133556186
Author: Bruice
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 71P
- a. Rank the following esters from most reactive to least reactive in the first slow step of a nucleophilic acyl substitution reaction (formation of the tetrahedral intermediate):
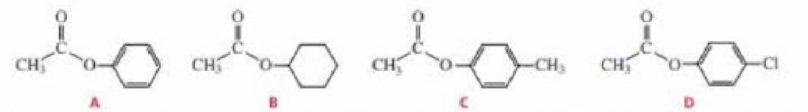
- b. Rank the same esters from most reactive to least reactive in the second slow step of a nucleophilic acyl substitution reaction (collapse of the tetrahedral intermediate).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate similarities and differences between natural, exchanged and pillared clays.
Show work. don't give Ai generated solution
In intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
Ch. 19.1 - Draw the structure for each of the following: a....Ch. 19.2 - PROBLEM 2
If electrophilic addition to benzene is...Ch. 19.4 - Why does hydration inactivate FeBr3?Ch. 19.6 - Prob. 5PCh. 19.7 - Prob. 6PCh. 19.8 - What is the major product of a Friedel-Crafts...Ch. 19.10 - Describe two ways to prepare each of the following...Ch. 19.12 - Prob. 9PCh. 19.13 - Name the following:Ch. 19.13 - Prob. 12P
Ch. 19.13 - Prob. 13PCh. 19.13 - Prob. 14PCh. 19.14 - Prob. 15PCh. 19.14 - List the compounds in each set from most reactive...Ch. 19.15 - Prob. 18PCh. 19.15 - What product(s) result from nitration of each of...Ch. 19.15 - Prob. 20PCh. 19.16 - Which acid in each of the following pairs is...Ch. 19.16 - Prob. 23PCh. 19.16 - Prob. 24PCh. 19.18 - Show how the following compounds can be...Ch. 19.18 - Give the products, if any, of each of the...Ch. 19.19 - a. Does a coupling reaction have to be used to...Ch. 19.19 - PROBLEM 28
Show how each of the following...Ch. 19.20 - What is the major product(s) of each of the...Ch. 19.20 - Prob. 30PCh. 19.21 - Why isn't FeBr3 used as a catalyst in the first...Ch. 19.21 - Prob. 33PCh. 19.21 - Write the sequence of steps required for the...Ch. 19.21 - Prob. 35PCh. 19.22 - What product is formed from reaction of...Ch. 19.22 - Prob. 37PCh. 19.22 - Draw the structure of the activated ring and the...Ch. 19.23 - Prob. 39PCh. 19.23 - Prob. 40PCh. 19.23 - Diazomethane can be used to convert a carboxylic...Ch. 19.24 - Prob. 42PCh. 19.24 - Prob. 43PCh. 19.24 - Prob. 44PCh. 19.25 - Prob. 45PCh. 19 - Draw the structure for each of the following: a....Ch. 19 - Prob. 47PCh. 19 - Prob. 48PCh. 19 - Prob. 49PCh. 19 - For each of the statements in Column I, choose a...Ch. 19 - What product is obtained from the reaction of...Ch. 19 - Draw the product(s) of each of the following...Ch. 19 - Rank the following substituted anilines from most...Ch. 19 - Prob. 54PCh. 19 - The compound with the 1H NMR spectrum shown below...Ch. 19 - Prob. 56PCh. 19 - Show how the following compounds can be...Ch. 19 - Prob. 58PCh. 19 - Rank each group of compounds from most reactive to...Ch. 19 - Prob. 60PCh. 19 - Describe two ways to prepare anisole from benzene.Ch. 19 - For each of the following components, indicate the...Ch. 19 - Prob. 63PCh. 19 - Prob. 64PCh. 19 - Prob. 65PCh. 19 - Prob. 66PCh. 19 - An aromatic hydrocarbon with a molecular formula...Ch. 19 - The following tertiary alkyl bromides undergo an...Ch. 19 - Show how the following compounds can be...Ch. 19 - Use the four compounds shown below to answer the...Ch. 19 - a. Rank the following esters from most reactive to...Ch. 19 - A mixture of 0.10 mol benzene and 0.10 mol...Ch. 19 - Prob. 73PCh. 19 - Benzene underwent a Friedel-Crafts acylation...Ch. 19 - Prob. 75PCh. 19 - Prob. 76PCh. 19 - Prob. 77PCh. 19 - Friedel-Crafts alkylations can be carried out with...Ch. 19 - Show how the following compounds can be prepared...Ch. 19 - Prob. 80PCh. 19 - Prob. 81PCh. 19 - a. Describe four ways the following reaction can...Ch. 19 - Propose a mechanism for each of the following...Ch. 19 - Prob. 84PCh. 19 - Describe how naphthalene can he prepared from the...Ch. 19 - Prob. 86PCh. 19 - Using resonance contributors for the carbocation...Ch. 19 - Prob. 88PCh. 19 - When heated with chromic acid, compound A forms...Ch. 19 - Prob. 90PCh. 19 - Prob. 91PCh. 19 - What reagents are required to carry out the...Ch. 19 - Show how the following compounds can be prepared...Ch. 19 - An unknown compound reacts with ethyl chloride and...Ch. 19 - How can you distinguish the following compounds...Ch. 19 - P-Fluoronitrobenzene is more reactive toward...Ch. 19 - a. Explain why the following reaction leads to the...Ch. 19 - Describe how mescaline can be synthesized from...Ch. 19 - Propose a mechanism for the following reaction...Ch. 19 - Explain why hydroxide ion catalyzes the reaction...Ch. 19 - Propose a mechanism for each of the following...Ch. 19 - Prob. 102PCh. 19 - Prob. 103PCh. 19 - Describe how 3-methyl-1-phenyl-3-pentanol can he...Ch. 19 - a. How can aspirin be synthesized from benzene? b....Ch. 19 - Prob. 106PCh. 19 - Show how Novocain, a painkiller used frequently by...Ch. 19 - Prob. 108PCh. 19 - Saccharin, an artificial sweetener, is about 300...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward7. Draw the mechanism to describe the following transformation: Note: This is a base catalyzed reaction. So, the last steps must make [OH]- OH [OH]¯ OH Heat Oarrow_forwardShow work with explanation...don't give Ai generated solutionarrow_forward
- Br. , H+ .OH Mg ether solvent H+, H₂O 17. Which one of the compounds below is the final product of the reaction sequence shown above? HO A HO HO OH D B OH HO OH C OH HO OH Earrow_forward8:57 PM Sun Jan 26 Content ← Explanation Page X Content X ALEKS Jade Nicol - Le A https://www-av C www-awa.aleks.com O States of Matter Understanding consequences of important physical properties of liquids ? QUESTION Liquid A is known to have a lower viscosity and lower surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment Liquid A and Liquid B are each pumped through tubes with an inside diameter of 27.0 mm, and the pressures PA and PB needed to produce a steady flow of 2.4 mL/s are measured. 25.0 mL of Liquid A are poured into a beaker, and 25.0 mL of Liquid B are poured into an identical beaker. Stirrers in each beaker are connected to motors, and the forces FA and FB needed to stir each liquid at a constant rate are measured. predicted outcome OPA will be greater than PB OPA will be less than PB OPA will be equal to PB It's impossible to predict whether PA or PB will be greater without more information.…arrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- 5. Please draw in the blanks the missing transition states and the correlated products. Explicitly display relevant absolute stereochemical configuration. MeOH I OMe H Endo transition state, dienophile approaching from the bottom of diene + H ཎྞཾ ཌཱརཱ༔,_o OMe H H OMe Endo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) + Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) MeO H H MeO H MeO H MeO H Harrow_forwardH H (1) H C. C C .H (2) (3) Cl H The ideal value for bond angle (1) is (Choose one) and the ideal value for bond angle (3) is (Choose one) degrees, the value for bond angle (2) is (Choose one) degrees, degrees.arrow_forwardShow work.....don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License