
Concept explainers
(a)
Interpretation: The number of coordinate bond formed by acetylacetone (acacH)chelating ligand needs to be determined.
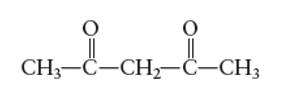
Concept Introduction: A coordination complex is composed of two main parts; metal atom/ ion and ligand. Ligands are the atom/ group of atom/ ion that can donate its extra pair of electrons to the electron deficient metal cation. It leads to the formation of coordinate bond between metal ion and ligand.
(b)
Interpretation: The number of coordinate bond formed by diethylenetriamine chelating ligand needs to be determined.
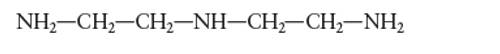
Concept Introduction: A coordination complex is composed of two main parts; metal atom/ ion and ligand. Ligands are the atom/ group of atom/ ion that can donate its extra pair of electrons to the electron deficient metal cation. It leads to the formation of coordinate bond between metal ion and ligand.
(c)
Interpretation: The number of coordinate bond formed by salen chelating ligand needs to be determined.
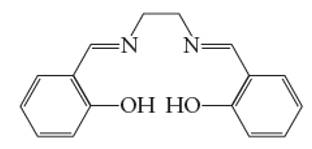
Concept Introduction: A coordination complex is composed of two main parts; metal atom/ ion and ligand. Ligands are the atom/ group of atom/ ion that can donate its extra pair of electrons to the electron deficient metal cation. It leads to the formation of coordinate bond between metal ion and ligand.
(d)
Interpretation: The number of coordinate bond formed by porphine chelating ligand needs to be determined.
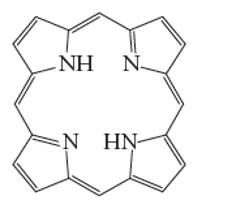
Concept Introduction: A coordination complex is composed of two main parts; metal atom/ ion and ligand. Ligands are the atom/ group of atom/ ion that can donate its extra pair of electrons to the electron deficient metal cation. It leads to the formation of coordinate bond between metal ion and ligand.
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Chemical Principles
- drawing, no aiarrow_forwardDraw the major organic product when each of the bellow reagents is added to 3,3-dimethylbutere. ✓ 3rd attempt Part 1 (0.3 point) H.C CH CH + 1. BHG THF 210 NaOH NJ 10000 Part 2 (0.3 point) HC- CH HC 2741 OH a Search 1. He|DA HO 2. NIBH さ 士 Ju See Periodic Table See Hint j = uz C H F F boxarrow_forwardSynthesis of 2-metilbenzimidazol from 1,2-diaminobenceno y propanona.arrow_forward
- Predict the product of the following reaction. 1st attempt HI 1 product 50300 Jul See Periodic Table See Hint P Br 石尚 Iarrow_forwardIndicate the substitutes in one place, if they are a diazonio room.arrow_forwardIndicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forward
- Indicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardSynthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning


