
Sketch and label a galvanic cell that makes use of the following spontaneous
Write the half-reactions for the anode and cathode. Give the standard cell notation. (Hint: Determine which half-reaction represents oxidation and which represents reduction.)
Interpretation:
For the given spontaneous redox reaction, a galvanic cell is to be labeled and sketched, the half- reactions for anode and cathode are to be written, and a standard cell notation is to be given.
Concept Introduction:
The electrode at which reduction occurs is known as cathode and the electrode at which oxidation occurs is known as anode.
The experimental apparatus that creates electric current from the spontaneous redox reaction is known as galvanic cell.
In the standard cell notation, the single vertical line represents the phase boundary and the double vertical line represents the salt bridge, the anode half-cell is described to the left of the double line and the cathode half-cell is described to the right of the double line.
Answer to Problem 1PE
Solution:
Cathode:
Anode:
Cell notation:
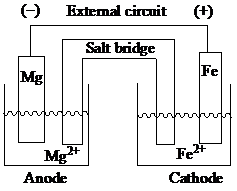
Explanation of Solution
The given spontaneous redox reaction is as:
At anode, oxidation takes place, whereas, at cathode, reduction takes place. In this galvanic cell, magnesium acts as the anode and iron acts as the cathode. In order to balance the redox reaction, iron gains two electrons while magnesium loses two electrons. Salt bridge enables the flow of ions which neutralize the charge imbalance. Within the salt bridge, the negative charge flows towards the anode to neutralize the accumulation of positive charge. Similarly, the positive charge flows towards the cathode to neutralize the accumulation of negative charge.
The half-cell reactions that take place at cathode and anode are as:
Half-cell reaction at cathode:
Half-cell reaction at anode:
The sketch of a galvanic cell is as:

In the standard cell notation, the magnesium anode half-cell is placed on the left and the cathode half-cell is placed on the right separated by the salt bridge. The standard cell notation for the given spontaneous redox reaction is represented as:
The half-cell reaction for anode and cathode and the standard notation cell notation have been written as:
Cathode:
Anode:
Cell notation:
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry: The Molecular Nature of Matter
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Applications and Investigations in Earth Science (9th Edition)
Living By Chemistry: First Edition Textbook
Campbell Essential Biology (7th Edition)
- On the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forwardRank the compounds below from lowest to highest melting point.arrow_forward18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forward
- someone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forwardThe dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forward
- Suppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forwardWhat mass of ethylene glycol, HOCH2CH2OH, Mustbe added to 5.50 kg of water to antifreeze that would work for the car radiator to -10.0 degrees celcius? MM (g/mol): 62.07arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





