
(a)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
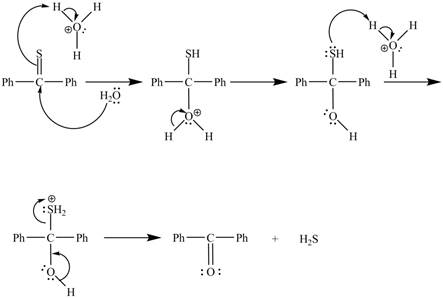
Explanation of Solution
The given reaction is shown below.
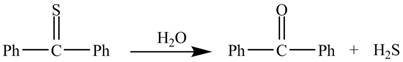
Figure 1
In the above reaction, diphenylmethanethione undergoes hydrolysis to yield benzophenone.
The curved arrow mechanism for the reaction is shown below.
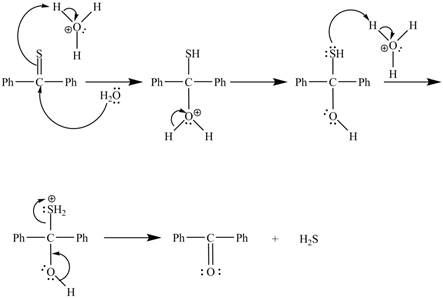
Figure 2
In the above mechanism, lone pairs of water attacks the
The curved arrow mechanism for the reaction is shown in Figure 2.
(b)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
The given reaction is shown below.

Figure 3
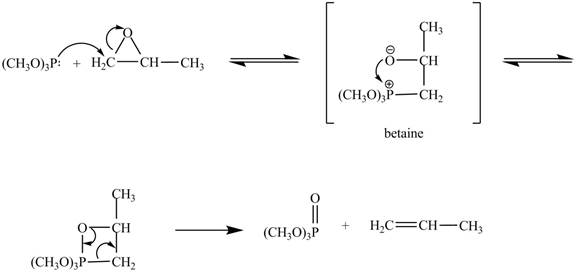
In the above reaction, trimethylphosphite reacts with
The curved arrow mechanism for the given reaction is shown below.
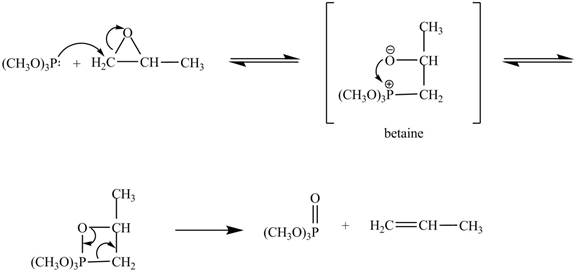
Figure 4
In the above mechanism, the trimethylphosphite reacts the least hindered site of the
The curved arrow mechanism for the given reaction is shown in Figure 4.
(c)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
The given reaction is shown below.
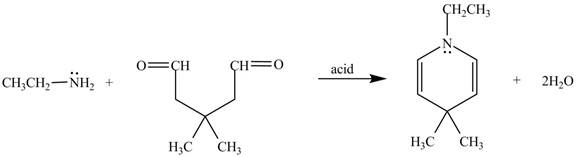
Figure 5
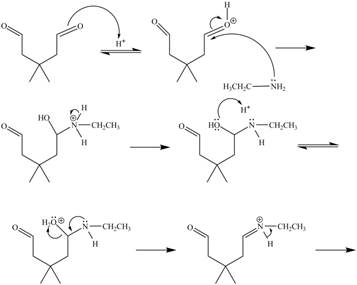
In the above reaction, ethylamine reacts with
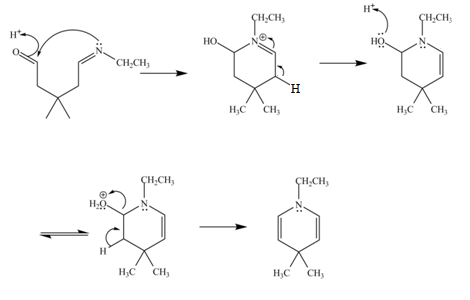
The curved arrow mechanism for the given reaction is shown below.
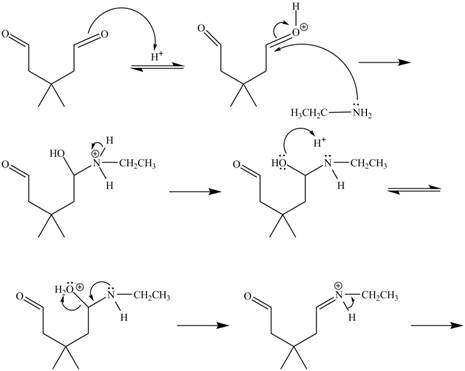

Figure 6
In the above mechanism, the carbonyl carbon of
The curved arrow mechanism for the given reaction is shown in Figure 6.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
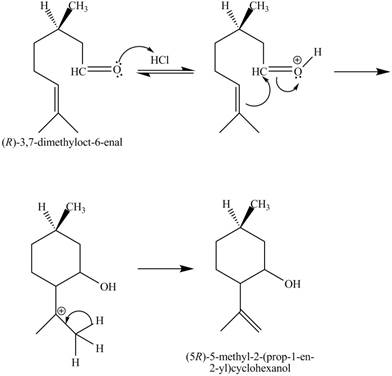
The given reaction is shown below.
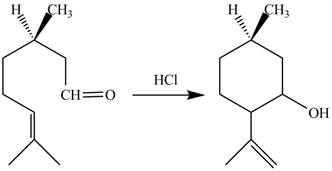
Figure 7
In the above reaction,
The curved arrow mechanim for the given reaction is shown below.
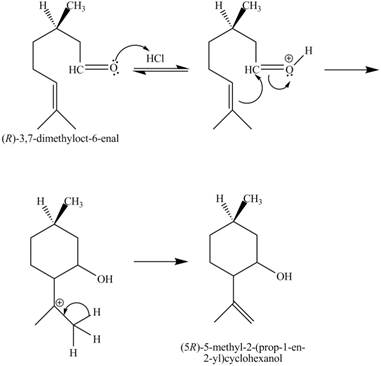
Figure 8
In the above mechanism, the carbonyl carbon of the compound
The curved arrow mechanism of the giveh reaction is shown in Figure 8.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
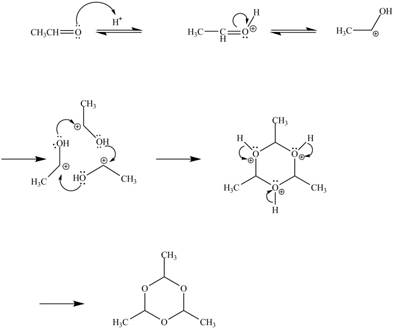
Explanation of Solution
The given reaction is shown below.
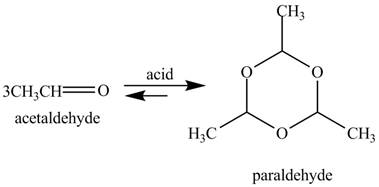
Figure 9
In the above reaction, acetaldehyde in the presence of acid catalyst yields a compound paraldehyde.
The curved arrow mechanism for the given reaction is shown below.
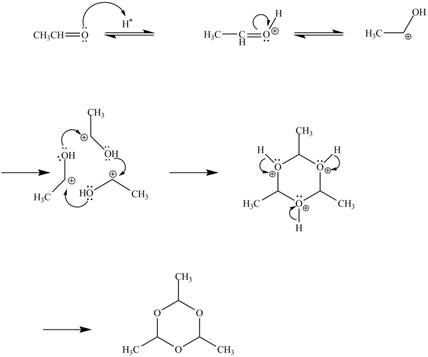
Figure 10
In the above mechanism, the acetaldehyde attacks the proton of acid catalyst to yield protonated acetaldehyde. The three moles of protonated acetaldehyde combines with each other to gives a cyclic structure. This cyclic structure on rearrangement gives the required product paraldehyde.
The curved arrow mechanism of the given reaction is shown in Figure 10.
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Draw the stepwise mechanismarrow_forwardDraw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forward
- Draw stepwise mechanismarrow_forwardPart I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forward
- What are the IUPAC Names of all the compounds in the picture?arrow_forward1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forward
- Li+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forwardQ4: Write organic product(s) of the following reactions and show the curved-arrow mechanism of the reactions. Br MeOH OSO2CH3 MeOHarrow_forwardProvide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
