
Concept explainers
(a)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
Oxime belongs to the family of imines. The formula of oxime is Unexpected text node: ' R '. Types of oxime are ketoxime and aldoxime. The preparation of oxime proceeds through a nucleophilic addition reaction. The catalyst acts as a nucleophile in the reaction.
Answer to Problem 19.48AP
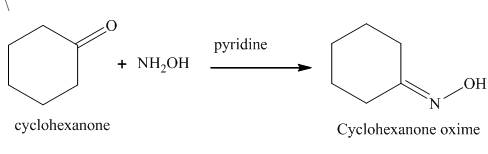
The product formed in the given reaction is cyclohexanone oxime in the presence of hydroxylamine and pyridine. The reaction is shown below.
Explanation of Solution
Cyclohexanone reacts with hydroxylamine to form the derivative of the oxime of the carbonyl compound. The nucleophilic addition takes place in the given reaction. The hydroxylamine is used as a nucleophile. The corresponding

Figure 1
The product formed in the given reaction is shown in Figure 1.
(b)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
The reaction between
Answer to Problem 19.48AP
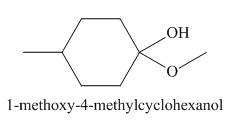
The product obtained in the given reaction is shown below.
Explanation of Solution
In the given reaction, 4- methyl-cyclohexanone reacts with methanol to give the product in the presence of p−toluene sulfonic acid. The name of the product is 1-methoxy-4-methylcyclohexanol. This is a nucleophilic substitution reaction.
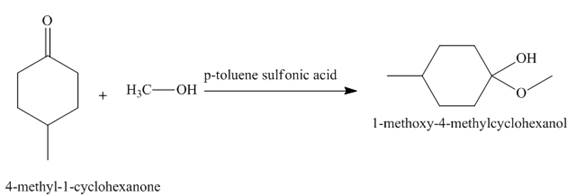
Figure 2
The treatment of 4- methyl-cyclohexanone with methanol to give the product in the presence of p−toluene sulfonic acid as shown in Figure 2.
(c)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
Ketones react with
Answer to Problem 19.48AP
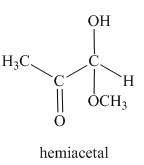
The product formed in the given reaction is a hemiacetal as shown below.
Explanation of Solution
In the third reaction, the methylglyoxal reacts with HCl in the presence of methanol. The keto group is less reactive than the aldehyde group. Therefore, substitution is takes place at the aldehydic position.
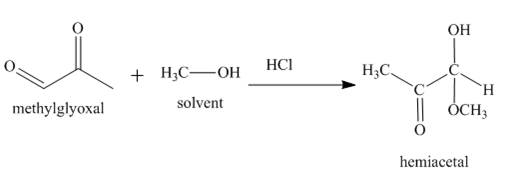
Figure 3
The reaction between methylglyoxal and HCl gives hemiacetal as the product as shown in Figure 3.
(d)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
The cyclic ethers are ring compounds which contain nitrogen, sulfur and oxygen groups within the ring. The easy method for synthesis of cyclic ether is Williamson synthesis.
Answer to Problem 19.48AP
The product formed when the 1, 9-dihydroxy-2, 8-dimethylnonan-5-one is reacted with substituted benzene is shown below.
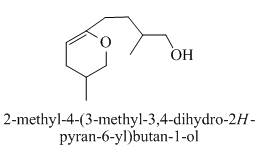
Explanation of Solution
The compound p-toluene sulfonic acid is also known as tosyl group which is act as a dehydrating agent.
When acid is treating with 1, 9-dihydroxy-2, 8-dimethylnonane-5-one then the product formed is a cyclic ether. The ring-closing takes place and form a cyclic ether as a product. The reaction proceeds in the presence of
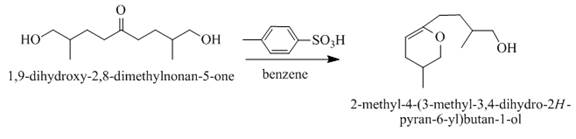
Figure 4
The product formed on the treatment of 1, 9-dihydroxy-2, 8-dimethylnonane-5-one with p-toluene sulfonic acid as shown in Figure 4.
(e)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
Grignard reagents are
Answer to Problem 19.48AP
The compound 1-Pheny-1-propan-1-one reacts with phenylmagnesium bromide to give the product shown below.
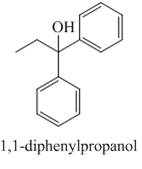
Explanation of Solution
The compound 1-Pheny-1-propan-1-one reacts with phenylmagnesium bromide followed by hydrolysis. The product formed is tertiary alcohol as a major product. The Grignard reagent acts as a reducing agent and H3O+ is used for hydrolysis.
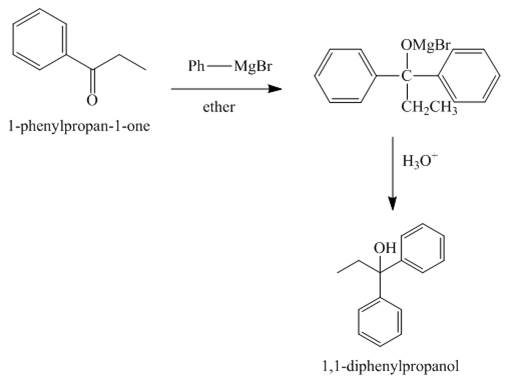
Figure 5
The reaction between 1-Pheny-1-propan-1-one and phenylmagnesium bromide is shown in Figure 5.
(f)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
The conversion of an aldehydic or ketonic group to alkene with the help of Wittig reagent is known as Wittig reaction.. The chemical name of Wittig reagent is triphenyl phosphonium ylide. The Wittig reagent gives good yields of alkene even when other
Answer to Problem 19.48AP
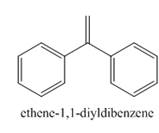
The product formed in the given reaction is ethene-1, 1-diylidibenzene as shown below.
Explanation of Solution
The carbonyl compounds react with Wittig reagent. The product formed is alkenes. In this Wittig reaction, most of the ketone reacts with triphenyl phosphonium ylide to generate an alkene and triphenylphosphine oxide as shown below.
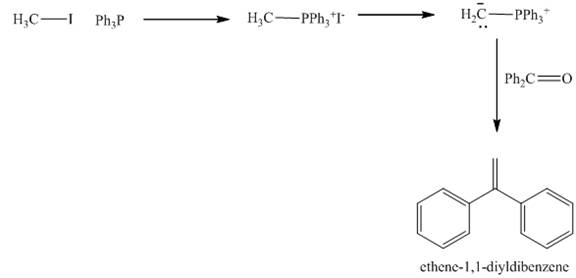
Figure 6
The treatment of haloalkane with triphenylphosphine gives the product ethene-1, 1-diylidibenzene as shown in Figure 6.
(g)
Interpretation:
The product formed in the given reaction is to be stated.
Concept introduction:
The Wittig reaction is the chemical reaction involves a change in an aldehyde or ketone converted into an alkene. The chemical name of Wittig reagent is triphenyl phosphonium ylide. The Wittig reagent gives good yields of alkene even when other functional groups are present on the aldehydes or ketone. However, as the steric hindrance of the aldehydes or ketone increases, the yield of alkene decreases.
Answer to Problem 19.48AP
The product formed in the given reaction is shown below.
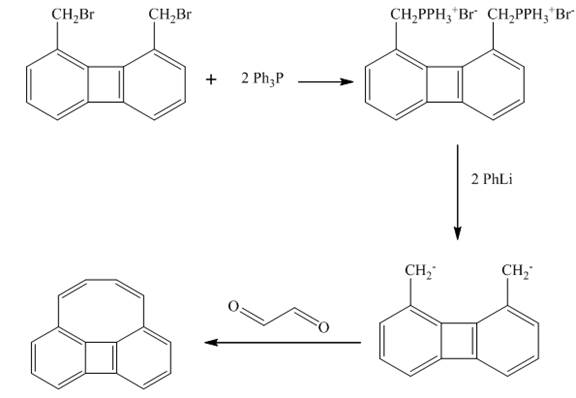
Explanation of Solution
The reaction is Wittig reaction. The reagent used is is triphenyl phosphonium ylide.
In the first step, the treatment of triphenyl phosphonium ylide reacts with 1, 8-bis(bromomethyl)biphenylene takes place. In the second step, the carbanion is formed in the presence of phenyllithium. In the final step, the carbanion formed in second step is treated with glyoxal to form the desired product. The product formed is (Z)-1, 4-dihydrocycloocta[def]biphenylene as shown below.

Figure 7
The given reaction is shown in Figure 7 in the presence of Wittig reagent.
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
