
(a)
Interpretation:
By using
Concept introduction:
For the analysis of the molecular formula of organic molecules, the sites of unsaturation, also known as IHD is used for the determination of the total number of ring and
In condensation reaction, the loss of water takes place.
Absence of
Absence of
Absence of
Answer to Problem 18.98P
Structure of the
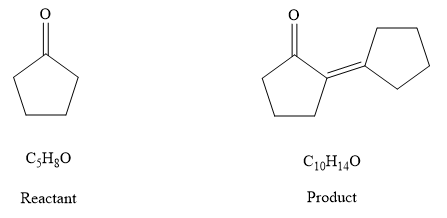
Explanation of Solution
IHD of
Comparing the formulas
The compound
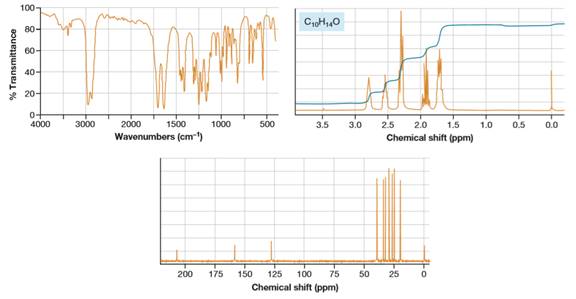
From the proton NMR, there is no alkene proton signal at
The NMR signal for the product is clear; there are five chemically distinct groups of protons. All the NMR signals in the product are
So, the structure of product is
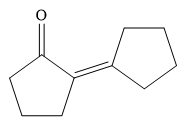
So, the reactant ketone with formula
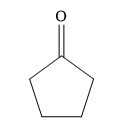
So, the structure of the

By using the given
(b)
Interpretation:
Mechanism of the given Aldol condensation reaction is to be determined.
Concept introduction:
In Aldol reaction,
In Aldol reaction, dimerization of an aldehyde or ketone to
For this reaction, the presence of
In the heterolysis step, water is removed and alkene (
Answer to Problem 18.98P
Mechanism of the given reaction is
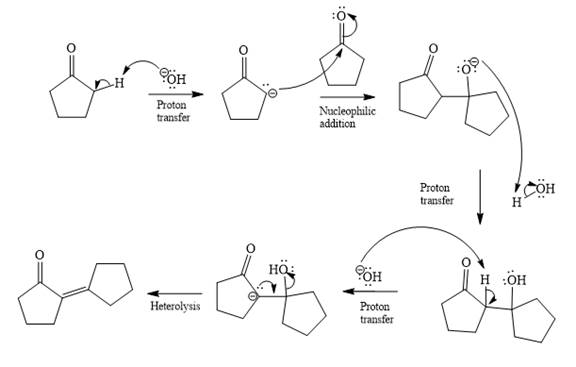
Explanation of Solution
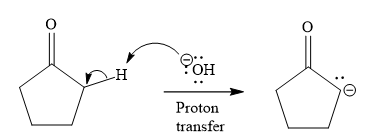
In the first step, the most acidic proton at alpha position in cyclopentanone is abstracted by the base. Hydroxide ion
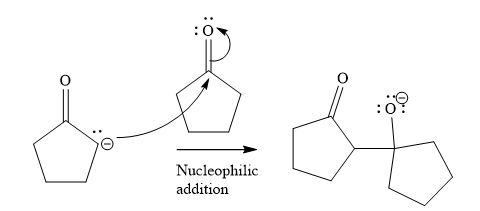
In the second step, nucleophilic addition takes place; the enolate anion acts as a nucleophile, and it will attack the second molecule of cyclopentanone. In this step, a new
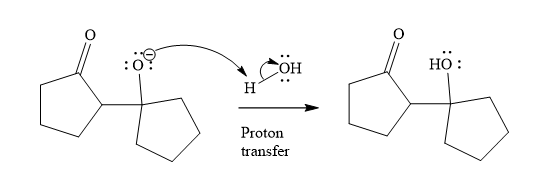
The third step is proton transfer.
In fourth step
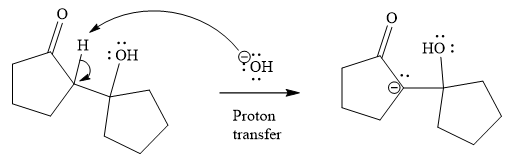
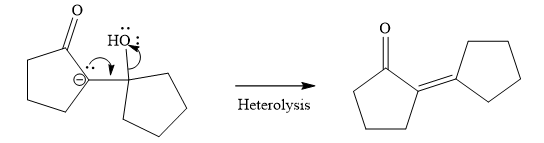
Last step of the reaction is heterolysis in which water is removed and
Complete and detailed mechanism for the given reaction is
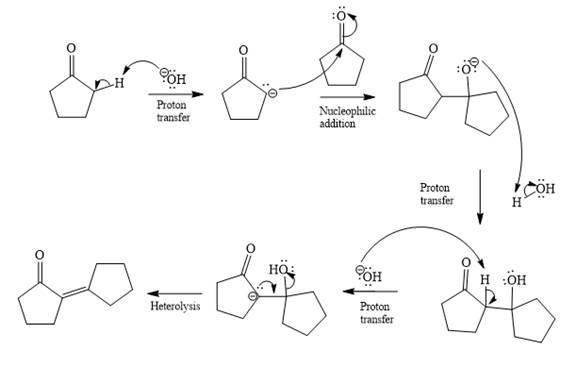
By using Aldol condensation reaction, the mechanism of the given reaction is determined.
Want to see more full solutions like this?
Chapter 18 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- You are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Using all the available information, set the upper and lower specification limits.arrow_forward43) 10.00 ml of vinegar (active ingredient is acetic acid) is titrated to the endpoint using 19.32 ml of 0.250 M sodium hydroxide. What is the molarity of acetic acid in the vinegar? YOU MUST SHOW YOUR WORK. NOTE: MA x VA = MB x VBarrow_forward424 Repon Sheet Rates of Chemical Reactions : Rate and Order of 1,0, Deception B. Effect of Temperature BATH TEMPERATURE 35'c Yol of Oh نام Time 485 Buret rend ing(n) 12 194 16. 6 18 20 10 22 24 14 115 95 14738 2158235 8:26 CMS 40148 Total volume of 0, collected Barometric pressure 770-572 ml mm Hg Vapor pressure of water at bath temperature (see Appendix L) 42.2 Slope Compared with the rate found for solution 1, there is Using the ideal gas law, calculate the moles of O; collected (show calculations) times faster 10 Based on the moles of O, evolved, calculate the molar concentration of the original 3% 1,0, solution (sho calculations)arrow_forward
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





