
(a)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and the major organic product is to be predicted.
Concept introduction:
When an
Answer to Problem 18.55P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
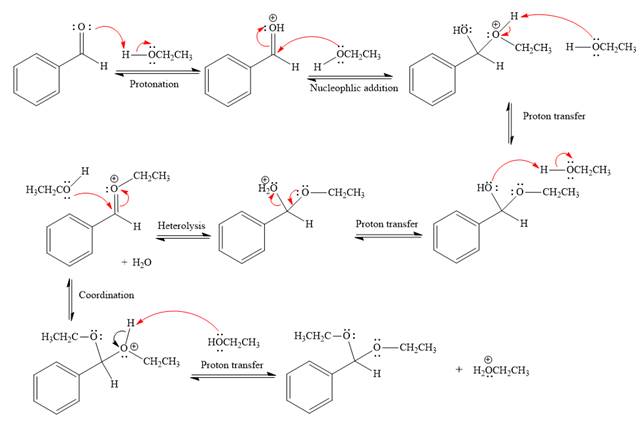
Explanation of Solution
The given reaction is
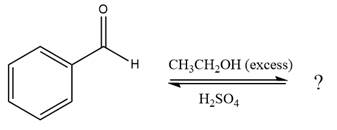
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess ethanol acts as the nucleophile.
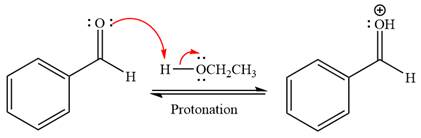
First three steps are acid catalyzed nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
Next, the weak nucleophile, alcohol, attacks the activated electrophilic carbon by nucleophilic addition reaction.
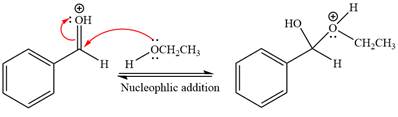
In the next step, deprotonation produces the uncharged hemiacetal.
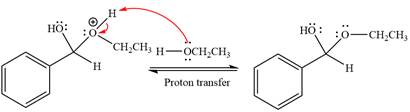
The remaining steps essentially make up
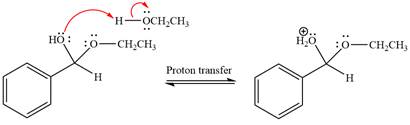
The
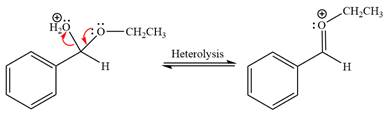
The resonance stabilized carbocation is further attacked by the ethyl alcohol nucleophile, which produces positively charged acetal.
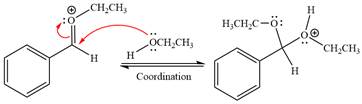
In the last step, the deprotonation of charged acetal by alcohol results in uncharged acetal
formation. Acetal is the major product of the given aldehyde.
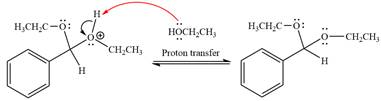
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is the major product.
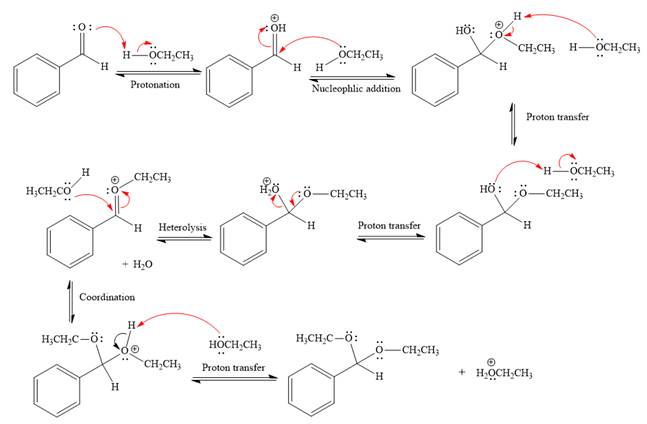
The complete, detailed mechanism of the given reaction under acidic medium and excess alcohol is drawn.
(b)
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and the major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups that are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reaction is carried forward by proton transfer and nucleophilic addition on the carbonyl carbon. An acetal is produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution requires the leaving group
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
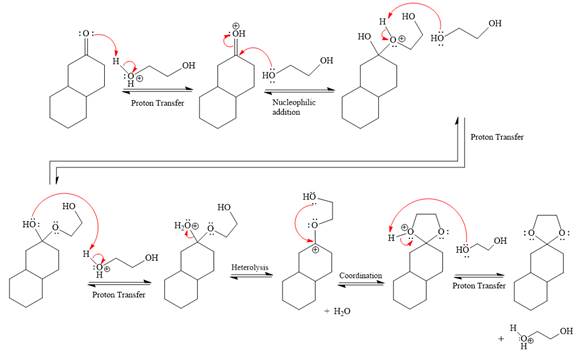
Explanation of Solution
The given reaction is
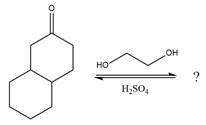
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess alcohol (
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
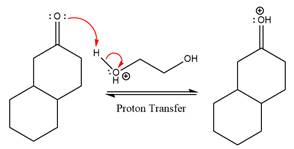
Next, the weak nucleophile,
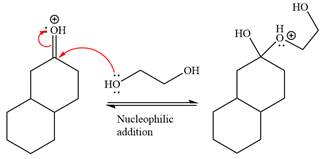
In the next step, deprotonation produces the uncharged hemiacetal.
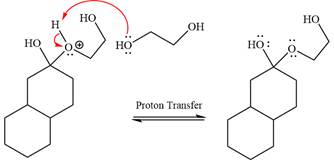
The remaining steps essentially make up a
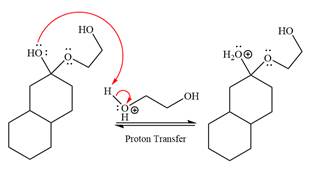
The
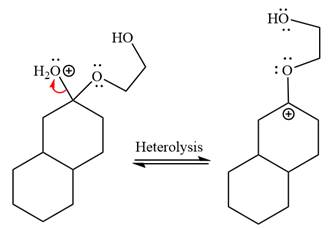
The resonance stabilized carbocation further attacked by the
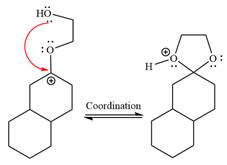
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
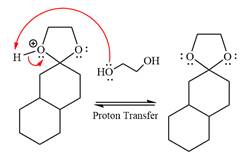
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
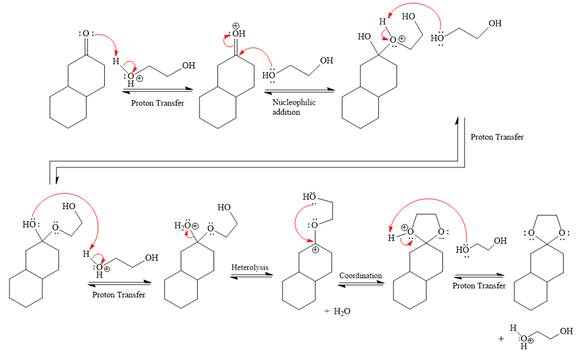
The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
(c)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reactions carried forward by the proton transfer and nucleophilic addition on the carbonyl carbon. An acetal produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution that requires the leaving group to be
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
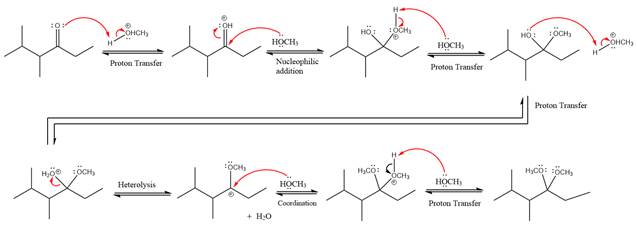
Explanation of Solution
The given reaction is
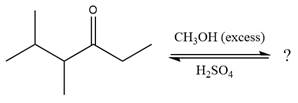
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess methanol acts as the nucleophile.
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
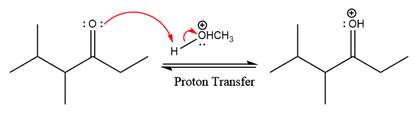
Next, the weak nucleophile, alcohol attacks on the activated electrophilic carbon by nucleophilic addition reaction.
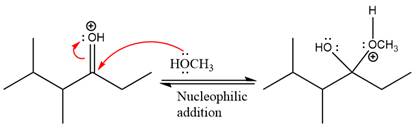
In the next step, deprotonation produces the uncharged hemiacetal.
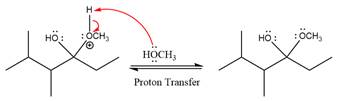
The remaining steps essentially make up a
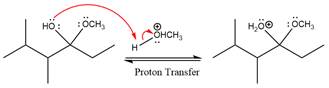
The
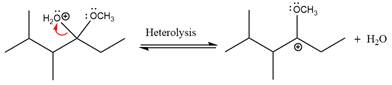
The resonance stabilized carbocation further attacked by the methyl alcohol nucleophile, which produced positively charged acetal.
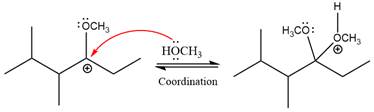
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
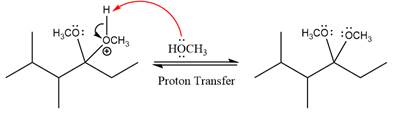
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
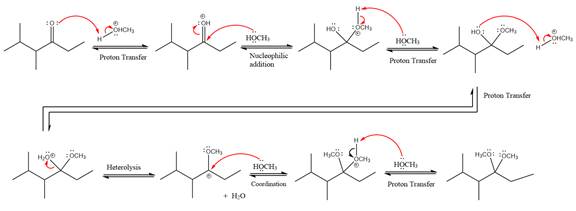
The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
(d)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reactions carried forward by the proton transfer and nucleophilic addition on the carbonyl carbon. An acetal produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution that requires the leaving group to be
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
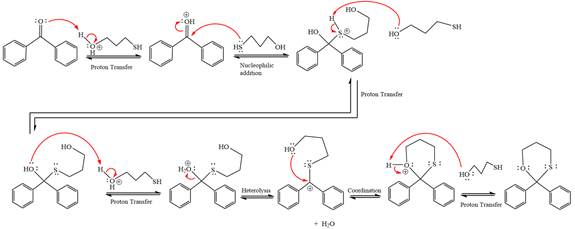
Explanation of Solution
The given reaction is
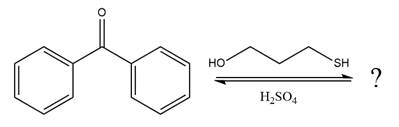
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
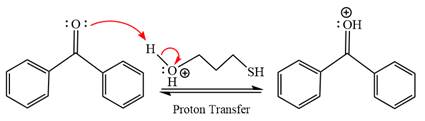
Next, the weak nucleophile, thiols attacks on the activated electrophilic carbon by nucleophilic addition reaction.
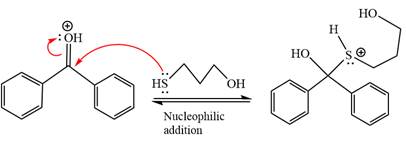
In the next step, deprotonation produces the uncharged hemiacetal.
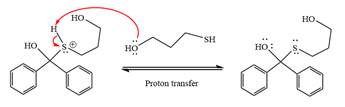
The remaining steps essentially make up a
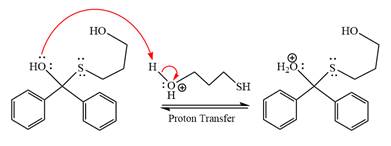
The
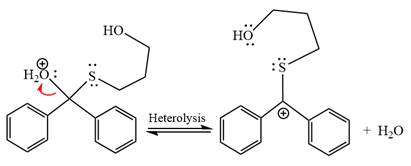
The resonance stabilized carbocation further attacked by the alcohol nucleophile, which produced positively charged acetal.
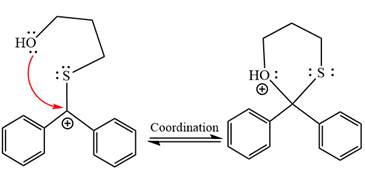
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
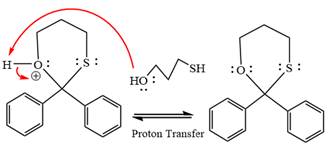
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
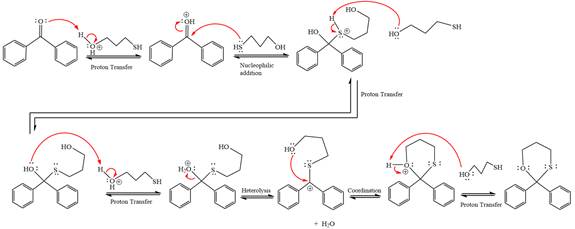
The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
(e)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reactions carried forward by the proton transfer and nucleophilic addition on the carbonyl carbon. An acetal produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution that requires the leaving group to be
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
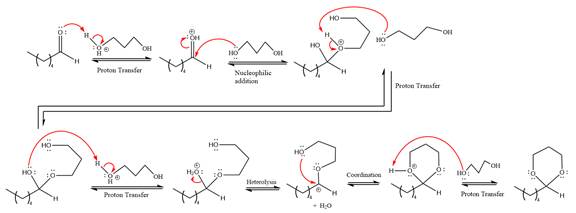
Explanation of Solution
The given reaction is
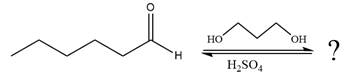
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
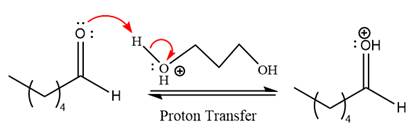
Next, the weak nucleophile,
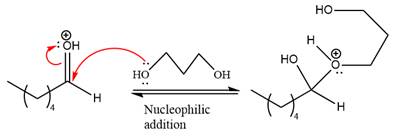
In the next step, deprotonation produces the uncharged hemiacetal.
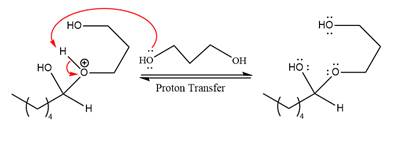
The remaining steps essentially make up a
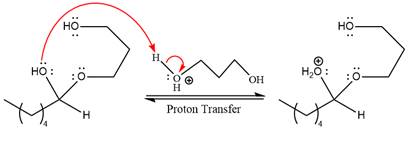
The
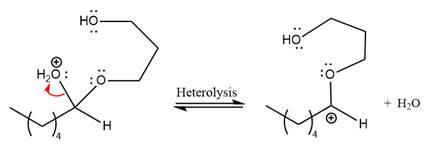
The resonance stabilized carbocation further attacked by the alcohol nucleophile, which produced positively charged acetal.
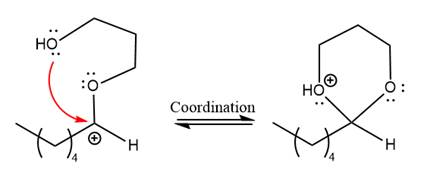
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
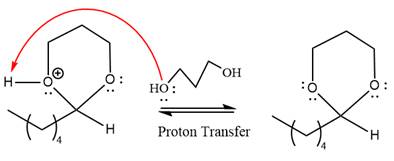
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.

The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
Want to see more full solutions like this?
Chapter 18 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- please help i cant find the article to even startarrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forward
- Predict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardPredict the major organic product for this reaction.arrow_forwardPredict the major organic product for this reaction.arrow_forward
- Predict the major organic product for this reaction.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





