
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.44SP
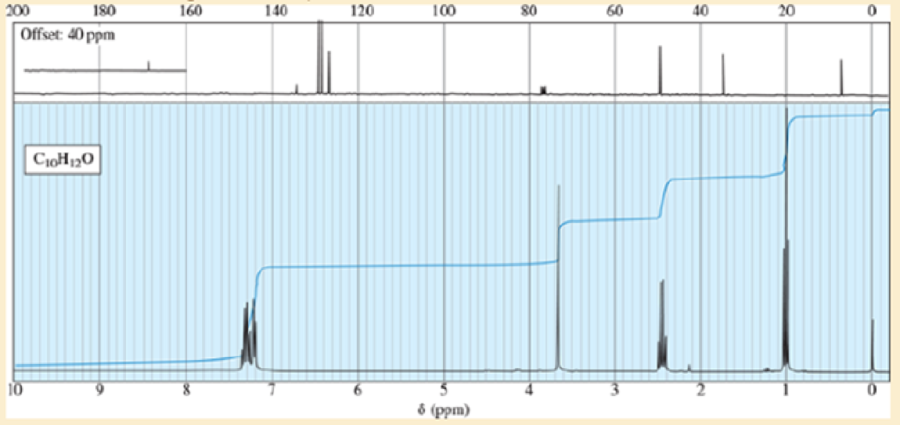
The proton NMR spectrum of a compound of formula C10H12O follows. This compound reacts with an acidic solution of 2,4-dinitrophenylhydrazine to give a crystalline derivative, but it gives a negative Tollens test. Propose a structure for this compound and give peak assignments to account for the signals in the spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The decomposition of dinitrogen pentoxide according to the equation:
50°C
2 N2O5(g)
4 NO2(g) + O2(g)
follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial
concentration of N2O5 is 0.275 M, determine:
the final concentration of N2O5 after 180 seconds.
...
Don't used hand raiting
CS2(g) →CS(g) + S(g)
The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹.
S
What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?
Chapter 18 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 18.3 - Give the IUPAC name and (if possible) a common...Ch. 18.5D - NMR spectra for two compounds are given here,...Ch. 18.5D - Why were no products from the McLafferty...Ch. 18.5D - Use equations to show the fragmentation leading to...Ch. 18.5E - Prob. 18.5PCh. 18.7D - Show how you would synthesize each compound from...Ch. 18.8 - Prob. 18.7PCh. 18.9 - Predict the products of the following reactions....Ch. 18.9 - Show how the following transformations may be...Ch. 18.10 - Prob. 18.10P
Ch. 18.11 - Show how you would accomplish the following...Ch. 18.11 - Prob. 18.12PCh. 18.12 - Propose mechanisms for a. the acid-catalyzed...Ch. 18.12 - Rank the following compounds in order of...Ch. 18.13 - Prob. 18.15PCh. 18.13 - Show how you would accomplish the following...Ch. 18.14 - Prob. 18.17PCh. 18.14 - Prob. 18.18PCh. 18.14 - Prob. 18.19PCh. 18.14 - Prob. 18.20PCh. 18.15 - 2,4-Dinitrophenylhydrazine is frequently used for...Ch. 18.15 - Prob. 18.22PCh. 18.15 - Prob. 18.23PCh. 18.16 - Prob. 18.24PCh. 18.16 - Prob. 18.25PCh. 18.16 - Show what alcohols and carbonyl compounds give the...Ch. 18.16 - In the mechanism for acetal hydrolysis shown, the...Ch. 18.16 - Prob. 18.28PCh. 18.17 - Show how you would accomplish the following...Ch. 18.18 - Prob. 18.30PCh. 18.18 - Prob. 18.31PCh. 18.18 - Prob. 18.32PCh. 18.18 - Show how Wittig reactions might be used to...Ch. 18.19 - Predict the major products of the following...Ch. 18.20C - Prob. 18.35PCh. 18.20C - Predict the major products of the following...Ch. 18 - Draw structures of the following derivatives. a....Ch. 18 - Prob. 18.38SPCh. 18 - Predict the major products of the following...Ch. 18 - Rank the following carbonyl compounds in order of...Ch. 18 - Acetals can serve as protecting groups for...Ch. 18 - Sketch the expected proton NMR spectrum of...Ch. 18 - A compound of formula C6H10O2 shows only two...Ch. 18 - The proton NMR spectrum of a compound of formula...Ch. 18 - The following compounds undergo McLafferty...Ch. 18 - An unknown compound gives a molecular ion of m/z...Ch. 18 - Show how you would accomplish the following...Ch. 18 - Prob. 18.48SPCh. 18 - Prob. 18.49SPCh. 18 - Propose mechanisms for the following reactions.Ch. 18 - Show how you would accomplish the following...Ch. 18 - Show how you would synthesize the following...Ch. 18 - Predict the products formed when cyclohexanone...Ch. 18 - Predict the products formed when...Ch. 18 - Show how you would synthesize octan-2-one from...Ch. 18 - Prob. 18.56SPCh. 18 - Both NaBH4 and NaBD4 are commercially available,...Ch. 18 - When LiAIH4 reduces 3-methylcyclopentanone, the...Ch. 18 - Prob. 18.59SPCh. 18 - Show how you would accomplish the following...Ch. 18 - There are three dioxane isomers 1,2-dioxane,...Ch. 18 - Two structures for the sugar glucose are shown on...Ch. 18 - Prob. 18.63SPCh. 18 - Prob. 18.64SPCh. 18 - Prob. 18.65SPCh. 18 - Prob. 18.66SPCh. 18 - Within each set of structures, indicate which will...Ch. 18 - Prob. 18.68SPCh. 18 - Prob. 18.69SPCh. 18 - Prob. 18.70SPCh. 18 - The UV spectrum of an unknown compound shows...Ch. 18 - a. Simple aminoacetals hydrolyze quickly and...Ch. 18 - The mass spectrum of unknown compound A shows a...Ch. 18 - Prob. 18.74SPCh. 18 - Prob. 18.75SPCh. 18 - Prob. 18.76SPCh. 18 - Prob. 18.77SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forwardControl Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forward
- Collagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forwardיווי 10 20 30 40 50 60 70 3.5 3 2.5 2 1.5 1 [ppm] 3.5 3 2.5 2 1.5 1 6 [ppm] 1 1.5 -2.5 3.5arrow_forward2H2S(g)+3O2(g)→2SO2(g)+2H2O(g) A 1.2mol sample of H2S(g) is combined with excess O2(g), and the reaction goes to completion. Question Which of the following predicts the theoretical yield of SO2(g) from the reaction? Responses 1.2 g Answer A: 1.2 grams A 41 g Answer B: 41 grams B 77 g Answer C: 77 grams C 154 g Answer D: 154 grams Darrow_forward
- Part VII. Below are the 'HNMR, 13 C-NMR, COSY 2D- NMR, and HSQC 2D-NMR (similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H1003 - Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 f1 (ppm) Predicted 13C NMR Spectrum 100 f1 (ppm) 30 220 210 200 190 180 170 160 150 140 130 120 110 90 80 70 -26 60 50 40 46 30 20 115 10 1.0 0.9 0.8 0 -10arrow_forwardQ: Arrange BCC and Fec metals, in sequence from the Fable (Dr. R's slides) and Calculate Volume and Density. Aa BCC V 52 5 SFCCarrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY